910554
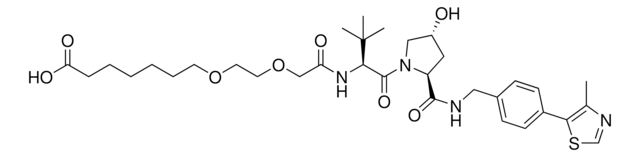
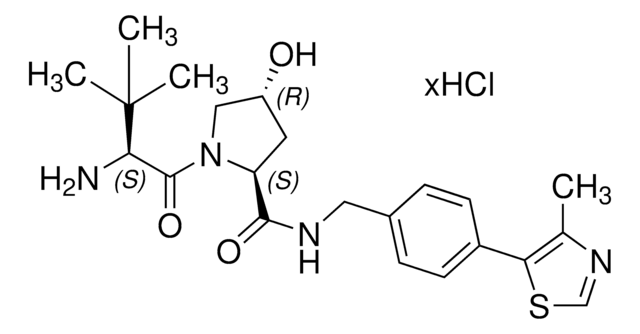
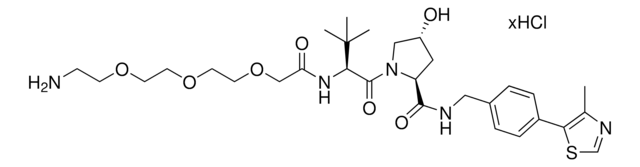
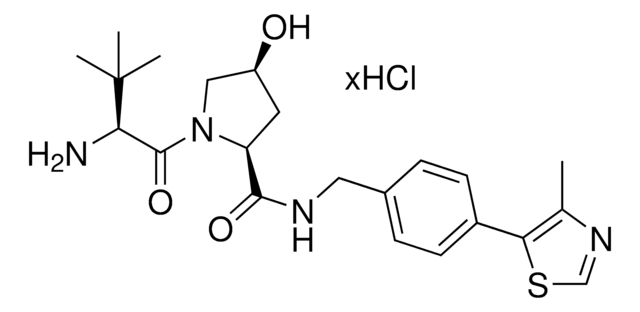
(S,R,S)-AHPC-PEG6-butyl CO2H
≥95%
别名:
(S)-3-((2S,4R)-4-Hydroxy-2-((4-(4-methylthiazol-5-yl)benzyl)carbamoyl)pyrrolidine-1-carbonyl)-2,2-dimethyl-5-oxo-7,10,13,16,19,22-hexaoxa-4-azanonacosan-29-oic acid, (S,R,S)-AHPC-2-2-2-2-2-2-6-Acid, Crosslinker−E3 ligase ligand conjugate, Protein degrader building block for PROTAC® research, Template for synthesis of targeted protein degrader, VH032 conjugate
About This Item
推荐产品
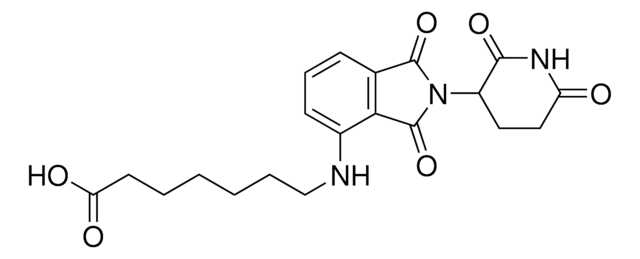
ligand
VH032
化驗
≥95%
形狀
(Liquid or Semi-Solid or Paste or Solid)
反應適用性
reactivity: amine reactive
reagent type: ligand-linker conjugate
官能基
carboxylic acid
儲存溫度
2-8°C
SMILES 字串
O=C(N[C@H](C(N1[C@H](C(NCC2=CC=C(C3=C(C)N=CS3)C=C2)=O)C[C@@H](O)C1)=O)C(C)(C)C)COCCOCCOCCOCCOCCOCCCCCCC(O)=O.Cl
應用
其他說明
Portal: Building PROTAC® Degraders for Targeted Protein Degradation
Modular PROTAC Design for the Degradation of Oncogenic BCR-ABL
Targeted Protein Degradation by Small Molecules
Small-Molecule PROTACS: New Approaches to Protein Degradation
Targeted Protein Degradation: from Chemical Biology to Drug Discovery
Impact of linker length on the activity of PROTACs
法律資訊
相關產品
儲存類別代碼
12 - Non Combustible Liquids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
商品
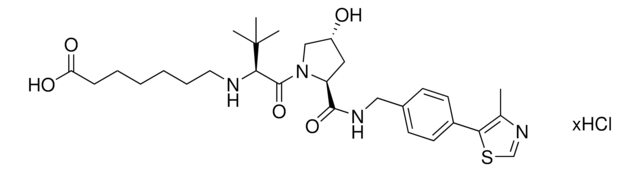
Partial PROTACs are a collection of crosslinker-E3 ligand conjugates with a pendant functional group for covalent linkage to a target ligand.
Partial PROTACs are a collection of crosslinker-E3 ligand conjugates with a pendant functional group for covalent linkage to a target ligand.
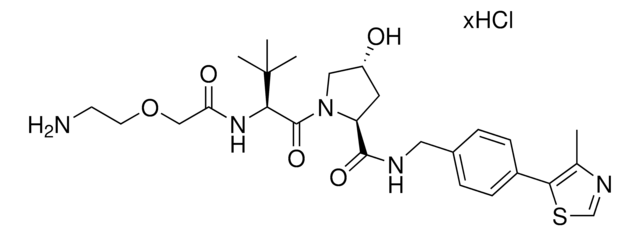
Partial PROTACs are a collection of crosslinker-E3 ligand conjugates with a pendant functional group for covalent linkage to a target ligand.
Partial PROTACs are a collection of crosslinker-E3 ligand conjugates with a pendant functional group for covalent linkage to a target ligand.
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门