推荐产品
化驗
95%
包含
phenothiazine as stabilizer
折射率
n20/D 1.4320
bp
47-49 °C/9 mbar
密度
0.894 g/mL at 25 °C
儲存溫度
2-8°C
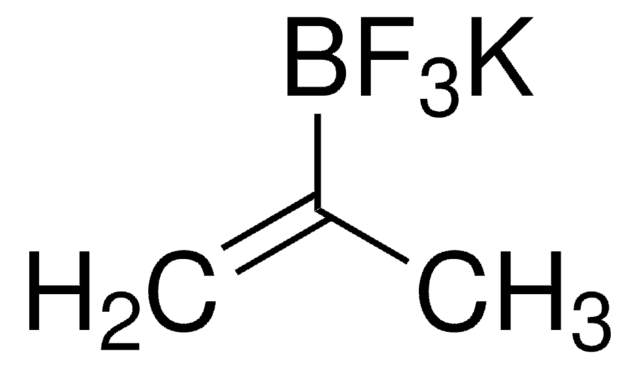
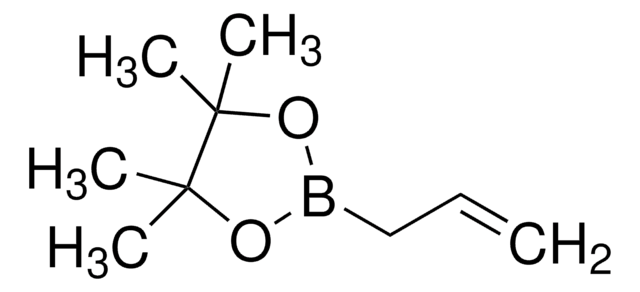
SMILES 字串
CC(=C)B1OC(C)(C)C(C)(C)O1
InChI
1S/C9H17BO2/c1-7(2)10-11-8(3,4)9(5,6)12-10/h1H2,2-6H3
InChI 密鑰
SVSUYEJKNSMKKW-UHFFFAOYSA-N
應用
Reagent used for
Reagent used in preparation of various therapeutic kinase and enzymatic inhibitors
- Palladium-catalyzed Suzuki-Miyaura cross-coupling processes
- Inverse-electron-demand Diels-Alder reaction
- Simmons-Smith Cyclopropanation Reaction
- Polyene cyclization
- Stereoselective aldol reactions
- Grubbs cross-metathesis reaction
- Intramolecular Suzuki-Miyaura reaction
- Stereoselective cross-metathesis
- Dipolar cycloaddition
- Iodosulfonylation
- Asymmetric conjugate addition and intramolecular hydroacylation
Reagent used in preparation of various therapeutic kinase and enzymatic inhibitors
Reagent used for
Reagent used in preparation of various therapeutic kinase and enzymatic inhibitors
- Palladium-catalyzed Suzuki-Miyaura cross-coupling reactions
- Inverse-electron-demand Diels-Alder reaction
- Simmons-Smith Cyclopropanation Reaction
- Polyene cyclization
- Stereoselective aldol reactions
- Grubbs cross-metathesis reaction
- Intramolecular Suzuki-Miyaura reaction
- Stereoselective cross-metathesis
- Dipolar cycloaddition
- Iodosulfonylation
- Asymmetric conjugate addition and intramolecular hydroacylation
Reagent used in preparation of various therapeutic kinase and enzymatic inhibitors
訊號詞
Warning
危險分類
Aquatic Chronic 3 - Eye Irrit. 2 - Flam. Liq. 3 - Skin Irrit. 2 - Skin Sens. 1 - STOT SE 3
標靶器官
Respiratory system
儲存類別代碼
3 - Flammable liquids
水污染物質分類(WGK)
WGK 3
閃點(°F)
107.6 °F
閃點(°C)
42 °C
個人防護裝備
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
其他客户在看
Total synthesis of apoptolidinone.
Bin Wu et al.
Angewandte Chemie (International ed. in English), 43(48), 6673-6675 (2004-12-14)
Synthesis of tri-substituted vinyl boronates via ruthenium-catalyzed olefin cross-metathesis
Morrill, C.; Funk, T. W.; Grubbs, R. H.
Tetrahedron Letters, 45, 7733-7736 (2004)
Halosulfonylation of unsaturated boronic esters: access to new electron-deficient alkenes and dienes
Guennouni, N.; et al.
Synlett, 7, 581-584 (1992)
The 1,3-dipolar cycloaddition of nitrile oxides to vinylboronic esters
Wallace, R. H.; Zong, K. K.; Schoene, M. P.
Current Topics in the Chemistry of Boron, 143, 78-81 (1994)
Facile Access to 3,5-Dihalogenated Pyrazoles by Sydnone Cycloaddition and their Versatile Functionalization by Pd-Catalyzed Cross-Coupling Processes
Delaunay, T.; et al.
European Journal of Organic Chemistry, 20-21, 3837-3848 (2011)
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门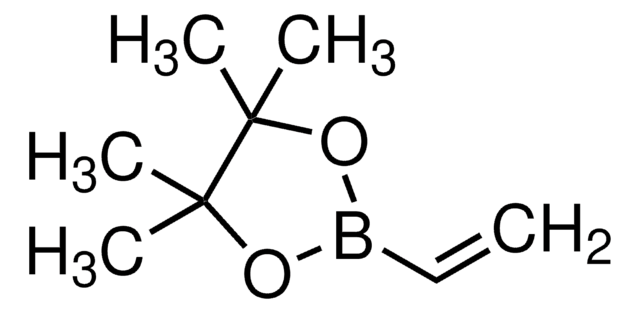
![[1,1′-双(二苯基膦)二茂铁]二氯化钯(II)](/deepweb/assets/sigmaaldrich/product/structures/130/734/8846aa26-1858-458a-998d-8c306c13bf0f/640/8846aa26-1858-458a-998d-8c306c13bf0f.png)