推荐产品
品質等級
化驗
95%
包含
phenothiazine as stabilizer
折射率
n20/D 1.4300 (lit.)
密度
0.908 g/mL at 25 °C (lit.)
儲存溫度
−20°C
SMILES 字串
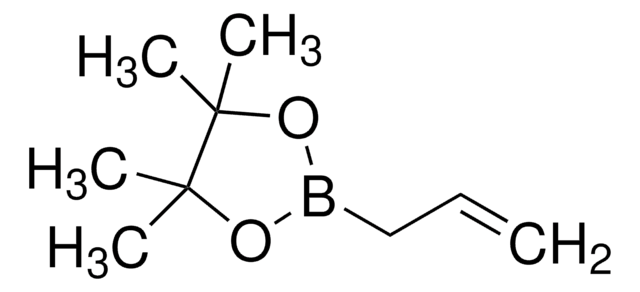
CC1(C)OB(OC1(C)C)C=C
InChI
1S/C8H15BO2/c1-6-9-10-7(2,3)8(4,5)11-9/h6H,1H2,2-5H3
InChI 密鑰
DPGSPRJLAZGUBQ-UHFFFAOYSA-N
應用
在“双”Heck-Mizoroki芳基化的使用中,生成β, β-二芳基化乙烯基硼酸盐,并与额外芳基卤化物反应形成Π-扩展系统。这种方法用于制备共轭树枝状化合物。 也用于通过光诱导黄原酸酯的添加制备γ羰基乙烯基硼酸盐。
试剂用于
制备过程中使用的试剂
- Suzuki-Miyaura 偶联反应
- Mizoroki-Heck 反应(级联反应)
- 分子内 Nozaki-Hiyama-Kishi 反应
- 立体选择性铜催化 γ-选择性和立体定向偶联
- 分子内 (4 + 1) 环加成反应的立体选择性控制及机理描述
- 金 (I) 催化卤代炔烃的氢磷氧基化反应和钯催化的连续交叉偶联反应合成三取代烯烃的区域和立体选择性
- 不对称桦木还原性烷基化
制备过程中使用的试剂
- 脂类感应分子管
- 酶抑制剂、抗生素、受体类似物和其他具有生物学意义的化合物(包括全合成)
訊號詞
Warning
危險聲明
危險分類
Aquatic Chronic 2 - Flam. Liq. 3 - Skin Sens. 1
儲存類別代碼
3 - Flammable liquids
水污染物質分類(WGK)
WGK 3
閃點(°F)
93.2 °F
閃點(°C)
34 °C
個人防護裝備
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
其他客户在看
Markus R Heinrich et al.
Chemical communications (Cambridge, England), (24), 3077-3079 (2005-06-17)
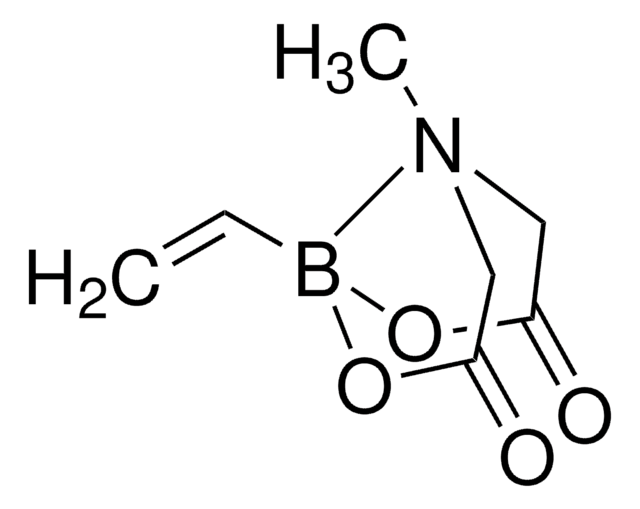
Gamma-carbonyl vinyl boronates can be prepared by a visible light induced radical chain addition of an S-acyl dithiocarbonate (xanthate) to the pinacol ester of vinyl boronic acid, followed by treatment with base.
Mingyu Yang et al.
Organic letters, 14(3), 816-819 (2012-01-20)
A Cu-catalyzed γ-selective coupling reaction between propargylic phosphates and aryl- or alkenylboronates afforded aryl- or alkenyl-conjugated allenes. The reaction showed excellent functional group compatibility in both the propargylic substrates and the boronates. The reaction of an enantioenriched propargylic phosphate proceeded
Francis Beaumier et al.
Journal of the American Chemical Society, 134(13), 5938-5953 (2012-03-13)
The stereoselective synthesis of 5-5, 6-5, and 7-5 fused O-heterocyclic compounds is reported. The key reaction is a formal intramolecular (4 + 1)-cycloaddition involving a dialkoxycarbene and an electron-deficient diene where the stereoselectivity is dependent on the length of the
Bathoju Chandra Chary et al.
Chemical communications (Cambridge, England), 47(27), 7851-7853 (2011-06-07)
A new stereoselective synthesis of trisubstituted alkenes is developed. Hydrophosphoryloxylation of haloalkynes provides Z-alkenyl halophosphates, which undergo Pd-catalyzed consecutive cross-coupling reactions to afford regio- and stereodefined trisubstituted alkenes.
Christopher A Leclair et al.
Tetrahedron letters, 51(52), 6852-6855 (2011-04-26)
A total synthesis of LL-Z1640-2 (2), a potent and selective kinase inhibitor, has been completed. The key step of the convergent synthesis utilized a late-stage intramolecular Nozaki-Hiyama-Kishi (NHK) reaction to close the macrocycle at the C6'-C7' bond.
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门
![[1,1′-双(二苯基膦)二茂铁]二氯化钯(II)](/deepweb/assets/sigmaaldrich/product/structures/130/734/8846aa26-1858-458a-998d-8c306c13bf0f/640/8846aa26-1858-458a-998d-8c306c13bf0f.png)
![[1,1′-双(二苯基膦)二茂铁]二氯化钯(II)二氯甲烷络合物](/deepweb/assets/sigmaaldrich/product/structures/825/986/4317978b-1256-4c82-ab74-6a6a3ef948b1/640/4317978b-1256-4c82-ab74-6a6a3ef948b1.png)