所有图片(2)
About This Item
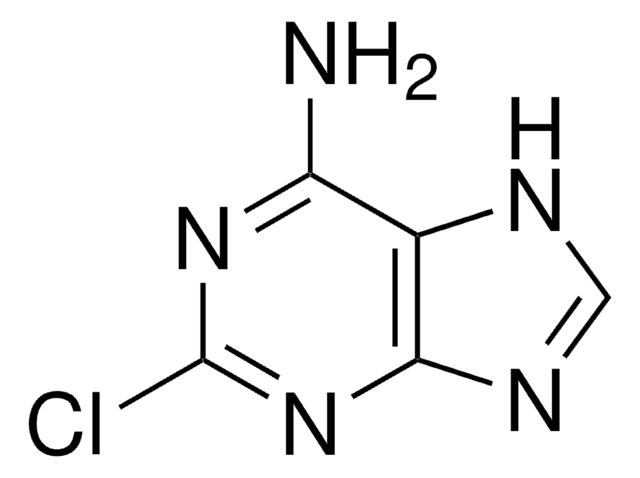
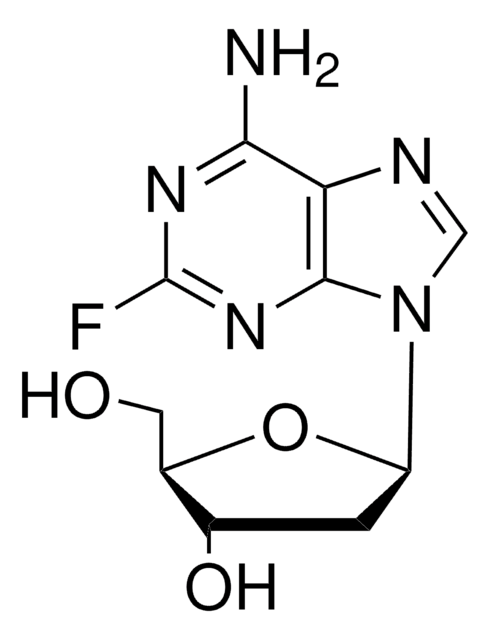
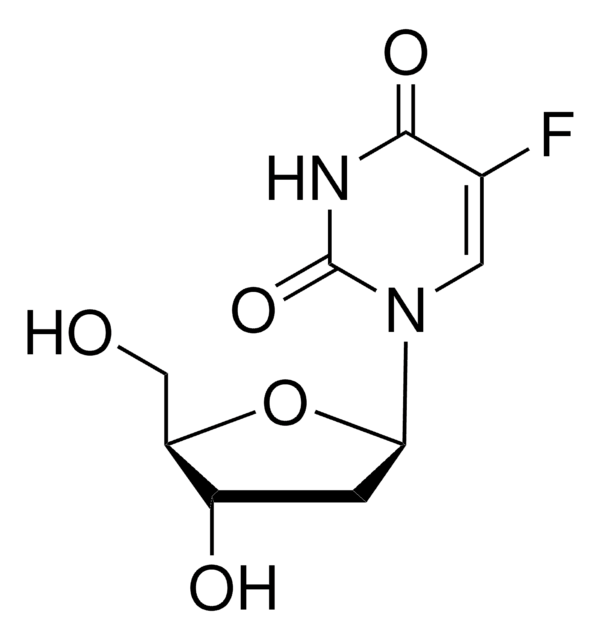
经验公式(希尔记法):
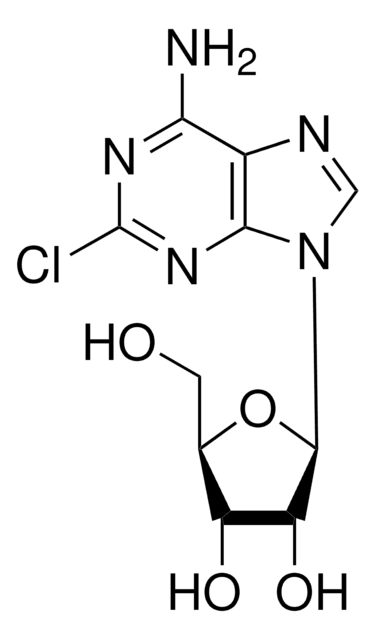
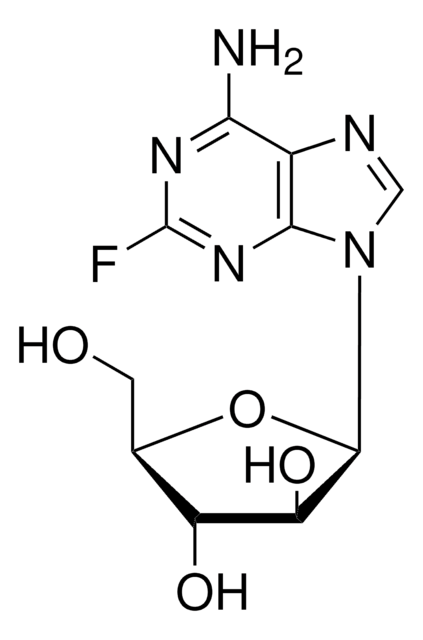
C10H12FN5O4
CAS号:
分子量:
285.23
MDL號碼:
分類程式碼代碼:
41106305
PubChem物質ID:
NACRES:
NA.22
推荐产品
品質等級
化驗
97%
形狀
solid
mp
240 °C (D) (lit.)
SMILES 字串
Nc1nc(F)nc2n(cnc12)[C@@H]3O[C@H](CO)[C@@H](O)[C@H]3O
InChI
1S/C10H12FN5O4/c11-10-14-7(12)4-8(15-10)16(2-13-4)9-6(19)5(18)3(1-17)20-9/h2-3,5-6,9,17-19H,1H2,(H2,12,14,15)/t3-,5-,6-,9-/m1/s1
InChI 密鑰
HBUBKKRHXORPQB-UUOKFMHZSA-N
基因資訊
human ... ADORA3(140)
rat ... Adora1(29290) , Adora2a(25369) , Adora3(25370)
訊號詞
Warning
危險分類
Acute Tox. 4 Oral - Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
標靶器官
Respiratory system
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
dust mask type N95 (US), Eyeshields, Faceshields, Gloves
U K Decking et al.
The American journal of physiology, 266(4 Pt 2), H1596-H1603 (1994-04-01)
Transport and phosphorylation of 2-fluoroadenosine (F-AR) were studied in human erythrocytes and porcine aortic endothelial cells by 19F-nuclear magnetic resonance (NMR) spectroscopy. F-AR (590 microM) added to a human erythrocyte suspension (15% hematocrit) was rapidly incorporated into adenine nucleotides at
M L Deras et al.
Biochemistry, 38(1), 303-310 (1999-01-16)
In contrast to several other glutamine amidotransferases including asparagine synthetase, cytidine 5'-triphosphate (CTP) synthetase, carbamoyl phosphate synthetase, and phosphoribosyl pyrophosphate (PRPP) amidotransferase, guanosine monophosphate synthetase (GMPS) will not utilize hydroxylamine as an alternative nitrogen source. Instead, the enzyme is inhibited
Larissa Romanello et al.
Acta crystallographica. Section D, Biological crystallography, 69(Pt 1), 126-136 (2013-01-01)
In adult schistosomes, the enzyme adenosine kinase (AK) is responsible for the incorporation of some adenosine analogues, such as 2-fluoroadenosine and tubercidin, into the nucleotide pool, but not others. In the present study, the structures of four complexes of Schistosoma
V B Berzin et al.
Bioorganicheskaia khimiia, 35(2), 210-214 (2009-06-20)
The preparative method for the synthesis of 2-fluoroadenosine starting from commercially available guanosine was developed. It included the intermediate formation of 2-amino-6-azido-9-(2,3,5-tri-O-acetyl-beta-D-ribofuranosyl)purine, which was isolated exclusively in the tetrazolo[5,1-i]-form {5-amino-7-(2,3,5-tri-O-acetyl-beta-D-ribofuranosyl)-7H-tetrazolo[5,1-i]purine}. The latter compound was converted by the Schiemann reaction to
Mian M Alauddin et al.
Nuclear medicine and biology, 34(3), 267-272 (2007-03-27)
Many fluorinated analogues of adenosine nucleoside have been synthesized and studied as potential antitumor and antiviral agents. Earlier, we reported radiosynthesis of 2'-deoxy-2'-[(18)F]fluoro-1-beta-D-arabinofuranosyl-adenine ([(18)F]-FAA) and 3'-deoxy-3'-[(18)F]fluoro-1-beta-d-xylofuranosyl-adenine ([(18)F]FXA). Now, we report their in vivo studies including blood clearance, biodistribution and micro-PET
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门