所有图片(1)
About This Item
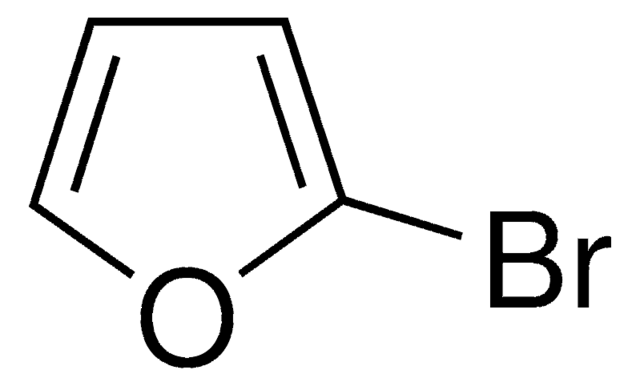
经验公式(希尔记法):
C8H5BrS
CAS号:
分子量:
213.09
MDL號碼:
分類程式碼代碼:
12352100
PubChem物質ID:
NACRES:
NA.22
推荐产品
品質等級
化驗
95%
折射率
n20/D 1.668 (lit.)
bp
269 °C/752.5 mmHg (lit.)
密度
1.629 g/mL at 25 °C (lit.)
官能基
bromo
SMILES 字串
Brc1csc2ccccc12
InChI
1S/C8H5BrS/c9-7-5-10-8-4-2-1-3-6(7)8/h1-5H
InChI 密鑰
SRWDQSRTOOMPMO-UHFFFAOYSA-N
一般說明
3-Bromothianaphthene is a heteroaryl halide. It undergoes Suzuki-Miyaura reaction with phenylboronic acid (PBA) or 3-thienylboronic acid in the presence of a novel heterogeneous Pd catalyst [Pd@PDEB, PDEB=poly(1,3-diethynylbenzene)]. The substitution reaction of 3-bromothianaphthene with piperidine to form 3-piperidinothianaphthene as the major product has been reported.
應用
3-Bromothianaphthene may be used in the synthesis of (benzo[b]thiophen-3-yl)trimethylstannane.
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
Eyeshields, Gloves, type N95 (US)
其他客户在看
Reusable, Highly Active Heterogeneous Palladium Catalyst by Convenient Self-Encapsulation Cross-Linking Polymerization for Multiple Carbon-Carbon Cross-Coupling Reactions at ppm to ppb Palladium Loadings.
Dong Z and Ye Z.
Advanced Synthesis & Catalysis, 356(16), 3401-3414 (2014)
Reactions of bromothianaphthenes with piperidine. Reinvestigation.
Reinecke MG, et al.
The Journal of Organic Chemistry, 38(7), 1365-1367 (1973)
Electronic influence of the thienyl sulfur atom on the oligomerization of ethylene by cobalt (II) 6-(thienyl)-2-(imino) pyridine catalysis.
Bianchini C, et al.
Organometallics, 26(3), 726-739 (2007)
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门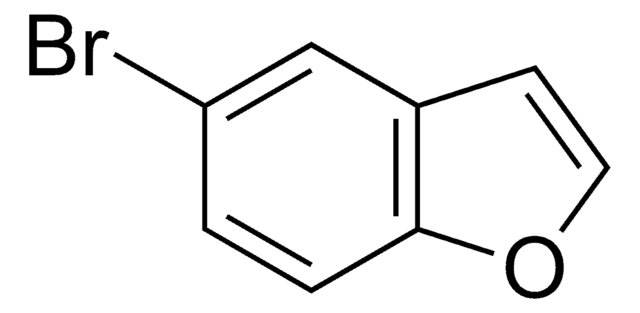
![6-Bromobenzo[b]thiophene 97%](/deepweb/assets/sigmaaldrich/product/structures/246/881/2541928a-1b0f-4e7e-93c0-1e30ed5b6b8a/640/2541928a-1b0f-4e7e-93c0-1e30ed5b6b8a.png)