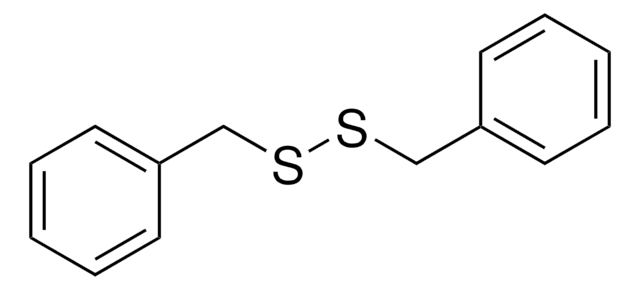
推荐产品
化驗
99%
形狀
powder
mp
58-60 °C (lit.)
溶解度
xylene: soluble 3%, clear, colorless to yellow
官能基
disulfide
SMILES 字串
S(Sc1ccccc1)c2ccccc2
InChI
1S/C12H10S2/c1-3-7-11(8-4-1)13-14-12-9-5-2-6-10-12/h1-10H
InChI 密鑰
GUUVPOWQJOLRAS-UHFFFAOYSA-N
正在寻找类似产品? 访问 产品对比指南
一般說明
二苯二硫醚被用作合成苯基硒硫化物(PhS-SePh)的前体,而PhS-SePh在锂离子电池的生产中起着重要的作用。
應用
苯基二硫化物是 dyfonate(杀虫剂)的水解产物 。苯基二硫化物(二苯基二硫化物)通过胺介导的单电子转移机制参与炔烃的加氢硫醚化反应 。
訊號詞
Warning
危險聲明
危險分類
Acute Tox. 4 Oral - Aquatic Acute 1 - Aquatic Chronic 1
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
dust mask type N95 (US), Eyeshields, Gloves
其他客户在看
Identification of hydrolytic metabolites of dyfonate in alkaline aqueous solutions by using high performance liquid chromatography-UV detection and gas chromatography-mass spectrometry.
Wang T, et al.
International Journal of Environmental Analytical Chemistry, 90(12), 948-961 (2010)
Tsuyoshi Taniguchi et al.
Organic letters, 11(15), 3298-3301 (2009-09-02)
Hydrothiolation of alkynes proceeds with diphenyl disulfide and tripropylamine. Amine-mediated single electron transfer to diphenyl disulfide can be proposed for the reaction mechanism. Applications of the method to radical cyclizations of eneyne compounds are also presented.
J M Young et al.
Agents and actions, 21(3-4), 314-315 (1987-08-01)
Indomethacin was administered subcutaneously to rats, 4 mg/kg/day for 4 consecutive days in order to produce erosions of the small intestine which were scored at necropsy on day 5. Orally administered phenidone (up to 250 mg/kg/day), a mixed cycloocygenase-lipoxygenase inhibitor
Y Ito et al.
Mutation research, 393(3), 307-316 (1997-12-11)
The suppressive effect of S-methyl methanethiosulfonate (MMTS) on aflatoxin B1 (AFB1)- or methyl methanesulfonate (MMS)-induced chromosome aberrations (CA) in rat bone marrow cells was studied. MMTS significantly suppressed CA induced by both AFB1 (an indirect-acting carcinogen) and MMS (a direct-acting
Y K Nakamura et al.
Mutation research, 385(1), 41-46 (1997-12-31)
S-Methyl methanethiosulfonate (MMTS) and diphenyl disulfide (DPDS) are temporary enzyme-sulfhydryl blocking agents. They are naturally occurring phytoalexin-like and synthetic substances known to be very potent bio-antimutagens in Escherichia coli B/r WP2. In the present paper, the suppressing effects of MMTS
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门