推荐产品
等級
purum
品質等級
化驗
≥97.0% (GC)
mp
59-61 °C (lit.)
60-63 °C
SMILES 字串
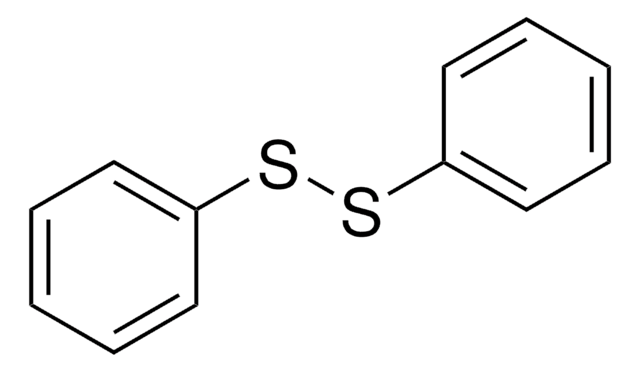
[Se]([Se]c1ccccc1)c2ccccc2
InChI
1S/C12H10Se2/c1-3-7-11(8-4-1)13-14-12-9-5-2-6-10-12/h1-10H
InChI 密鑰
YWWZCHLUQSHMCL-UHFFFAOYSA-N
正在寻找类似产品? 访问 产品对比指南
一般說明
Diphenyl diselenide (Ph2Se2) is an organoselenium compound. Its crystalline structure, toxicokinetic properties, antioxidant and anti-inflammatory activities have been investigated. Its ability to reverse the oxidative brain damage and mitochondrial dysfunction induced by acetaminophen has been studied in mice. It reacts with thallium (Tl) to form TI (SePh). It participates in a four-component radical coupling to form substituted cyclopentanes.
應用
Diphenyl diselenide (Ph2Se2) may be used in the following studies:
- methoxyselenenylation of alkenes
- dihydroxylation of double bonds
- hydrothiolation of terminal alkynes
- unsymmetrical diorganyl selenides
- 1-(phenylselenomethyl)vinyl selenides
- allylic phenyl selenides
其他說明
引入苯硒基的试剂,用于消去和氧化反应,综述
訊號詞
Danger
危險分類
Acute Tox. 3 Inhalation - Acute Tox. 3 Oral - Aquatic Acute 1 - Aquatic Chronic 1 - STOT RE 2
儲存類別代碼
6.1A - Combustible acute toxic Cat. 1 and 2 / very toxic hazardous materials
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
Eyeshields, Faceshields, Gloves, type P2 (EN 143) respirator cartridges
其他客户在看
Eco-Friendly Olefin Dihydroxylation Catalyzed by Diphenyl Diselenide.
Santoro S, et al.
Advanced Synthesis & Catalysis, 350(18), 2881-2884 (2008)
Samarium (II) di-iodide induced synthesis of allylic phenyl selenides from allylic acetates and diphenyl diselenide in the presence of palladium catalyst.
Fukuzawa S, et al.
Chemistry Letters (Jpn), 6, 927-930 (1990)
The reaction of diphenyl diselenide with peroxydisulphate ions in methanol a convenient procedure to effect the methoxyselenenylation of alkenes.
Tiecco M, et al.
Tetrahedron Letters, 30(11), 1417-1420 (1989)
Highly Selective Sequential Addition and Cyclization Reactions Involving Diphenyl Diselenide, an Alkyne, and Alkenes under Visible-Light Irradiation.
Tsuchii K, et al.
Angewandte Chemie (International Edition in English), 42(30), 3490-3493 (2003)
Photo-initiated addition of diphenyl diselenide to allenes.
Ogawa A, et al.
Tetrahedron Letters, 31(41), 5931-5934 (1990)
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门




![(Ir[dF(CF3)ppy]2(dtbpy))PF6](/deepweb/assets/sigmaaldrich/product/structures/982/913/02dd8ddd-6deb-40a0-ab9b-07b18f1abb09/640/02dd8ddd-6deb-40a0-ab9b-07b18f1abb09.png)