推荐产品
化驗
95%
形狀
liquid
折射率
n20/D 1.443 (lit.)
bp
107-110 °C/11 mmHg (lit.)
密度
1.063 g/mL at 25 °C (lit.)
官能基
ester
儲存溫度
2-8°C
SMILES 字串
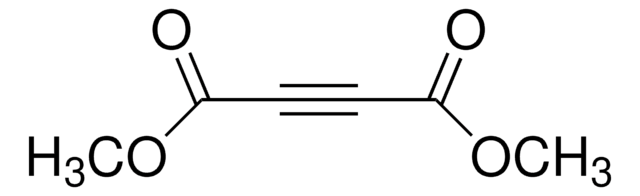
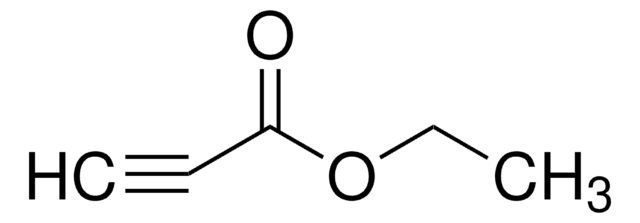
CCOC(=O)C#CC(=O)OCC
InChI
1S/C8H10O4/c1-3-11-7(9)5-6-8(10)12-4-2/h3-4H2,1-2H3
InChI 密鑰
STRNXFOUBFLVIN-UHFFFAOYSA-N
正在寻找类似产品? 访问 产品对比指南
一般說明
丁炔二酸二乙酯是一种蛋白交联剂。
丁炔二酸二乙酯可用于O-乙烯基肟合成的Michael受体,并用于亲核加成反应。
丁炔二酸二乙酯可用于O-乙烯基肟合成的Michael受体,并用于亲核加成反应。
應用
丁炔二酸二乙酯被用于合成:
- 3,4,5-三取代2(5H)-呋喃酮衍生物
- 高度官能化的噻唑烷酮衍生物
- 新型环状过氧化物葡萄糖苷
- 通过Diels-Alder反应合成4,11-dimesitylbisanthene,一种可溶性bisanthene衍生物
訊號詞
Danger
危險聲明
危險分類
Skin Corr. 1B
儲存類別代碼
8A - Combustible corrosive hazardous materials
水污染物質分類(WGK)
WGK 3
閃點(°F)
201.2 °F - closed cup
閃點(°C)
94 °C - closed cup
個人防護裝備
Faceshields, Gloves, Goggles, type ABEK (EN14387) respirator filter
其他客户在看
Eric H Fort et al.
Journal of the American Chemical Society, 131(44), 16006-16007 (2009-10-17)
A soluble bisanthene derivative, 4,11-dimesitylbisanthene, has been synthesized in three steps from bianthrone. In hot toluene, this bisanthene undergoes a clean Diels-Alder reaction with diethyl acetylenedicarboxylate to give a rearomatized 1:1 cycloadduct and, more slowly, a rearomatized 2:1 cycloadduct. In
W M Basyouni et al.
Drug research, 65(9), 473-478 (2014-09-11)
A series of 3,4,5-trisubstituted 2(5H)-furanone derivatives was synthesized through one-pot reaction of amines, aldehydes and diethyl acetylenedicarboxylate. Silica sulfuric acid efficiently catalyzes the 3-component reaction to afford the corresponding 2(5H)-furanones in high yields. The synthesized compounds were tested against HEPG2
Di-Zao Li et al.
Journal of Asian natural products research, 11(7), 613-620 (2010-02-26)
Four novel cyclic peroxide glucosides 15a, 15b, 16a, and 16b, optically pure analogs of shuangkangsu (1), which is an anti-virus natural product with an unusual skeleton isolated from the buds of Lonicera japonica Thunb, were first synthesized totally in six
Kh Mahid Uddin et al.
Dalton transactions (Cambridge, England : 2003), 46(39), 13597-13609 (2017-09-28)
The reactivity of the face-capped benzothiazolate clusters HOs
Abdelmadjid Benmohammed et al.
Molecules (Basel, Switzerland), 19(3), 3068-3083 (2014-03-13)
We present herein the synthesis in good yields of two series of highly functionalized thiazolidinone derivatives from the reactions of various 4-phenyl-3-thio-semicarbazones with ethyl 2-bromoacetate and diethyl acetylenedicarboxylate, respectively.
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系客户支持