推荐产品
蒸汽密度
9 (vs air)
品質等級
蒸汽壓力
5 mmHg ( 20 °C)
產品線
ReagentPlus®
化驗
99%
特點
achiral
反應適用性
reaction type: Buchwald-Hartwig Cross Coupling Reaction
reaction type: Heck Reaction
reaction type: Hiyama Coupling
reaction type: Negishi Coupling
reaction type: Sonogashira Coupling
reaction type: Stille Coupling
reaction type: Suzuki-Miyaura Coupling
reagent type: ligand
reaction type: Cross Couplings
參數
air stable
bp
377 °C (lit.)
mp
79-81 °C (lit.)
官能基
phosphine
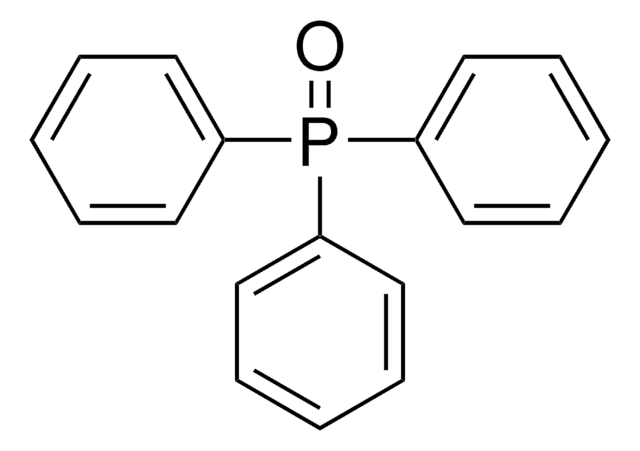
SMILES 字串
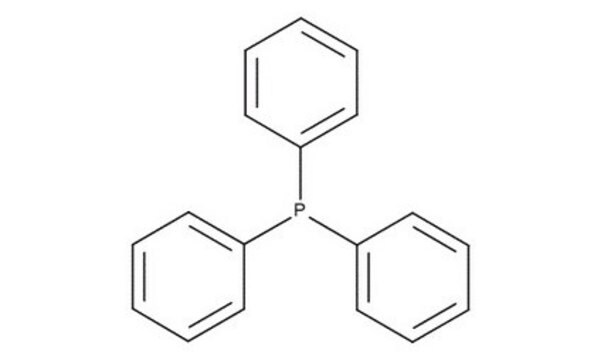
c1ccc(cc1)P(c2ccccc2)c3ccccc3
InChI
1S/C18H15P/c1-4-10-16(11-5-1)19(17-12-6-2-7-13-17)18-14-8-3-9-15-18/h1-15H
InChI 密鑰
RIOQSEWOXXDEQQ-UHFFFAOYSA-N
正在寻找类似产品? 访问 产品对比指南
一般說明
應用
法律資訊
訊號詞
Danger
危險分類
Acute Tox. 4 Oral - Eye Dam. 1 - Skin Sens. 1B - STOT RE 1 Inhalation
標靶器官
Central nervous system,Peripheral nervous system
儲存類別代碼
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
水污染物質分類(WGK)
WGK 2
閃點(°F)
356.0 °F - closed cup
閃點(°C)
180 °C - closed cup
個人防護裝備
dust mask type N95 (US), Eyeshields, Faceshields, Gloves
其他客户在看
商品
The Heck reaction is the palladium catalyzed cross-coupling reaction between alkenes and aryl or vinyl halides (or triflates) to afford substituted alkenes.
The Heck reaction is the palladium catalyzed cross-coupling reaction between alkenes and aryl or vinyl halides (or triflates) to afford substituted alkenes.
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门