推荐产品
品質等級
化驗
99%
折射率
n20/D 1.5002 (lit.)
bp
119-120 °C/18 mmHg (lit.)
密度
1.126 g/mL at 25 °C (lit.)
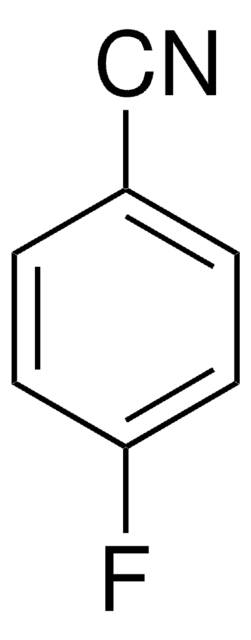
官能基
fluoro
nitrile
SMILES 字串
Fc1ccc(CC#N)cc1
InChI
1S/C8H6FN/c9-8-3-1-7(2-4-8)5-6-10/h1-4H,5H2
InChI 密鑰
JHQBLYITVCBGTO-UHFFFAOYSA-N
正在寻找类似产品? 访问 产品对比指南
一般說明
4-Fluorophenylacetonitrile is starting reagent in the synthesis of 1-Alkyl-N-[2-ethyl-2-(4-fluorophenyl)butyl]piperidine-4-carboxamide derivatives.
生化/生理作用
4-Fluorophenylacetonitrile undergoes biotransformation to 4-Fluorophenylacetic acid by marine fungi, Aspergillus sydowii Ce19.
訊號詞
Warning
危險分類
Acute Tox. 4 Dermal - Acute Tox. 4 Inhalation - Acute Tox. 4 Oral - Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
標靶器官
Respiratory system
儲存類別代碼
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
水污染物質分類(WGK)
WGK 3
閃點(°F)
226.4 °F - closed cup
閃點(°C)
108 °C - closed cup
個人防護裝備
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
其他客户在看
Susumu Watanuki et al.
Bioorganic & medicinal chemistry, 19(18), 5628-5638 (2011-08-31)
We synthesized and evaluated inhibitory activity against T-type Ca(2+) channels for a series of 1-alkyl-N-[2-ethyl-2-(4-fluorophenyl)butyl]piperidine-4-carboxamide derivatives. Structure-activity relationship studies have revealed that dialkyl substituents at the benzylic position play an important role in increasing inhibitory activity. Oral administration of N-[2-ethyl-2-(4-fluorophenyl)butyl]-1-(2-phenylethyl)piperidine-4-carboxamide
Julieta Rangel de Oliveira et al.
Marine biotechnology (New York, N.Y.), 15(1), 97-103 (2012-07-14)
Marine fungi belonging to the genera Aspergillus, Penicillium, Cladosporium, and Bionectria catalyzed the biotransformation of phenylacetonitrile to 2-hydroxyphenylacetic acid. Eight marine fungi, selected and cultured with phenylacetonitrile in liquid mineral medium, catalyzed it quantitative biotransformation to 2-hydroxyphenylacetic acid. In this
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门