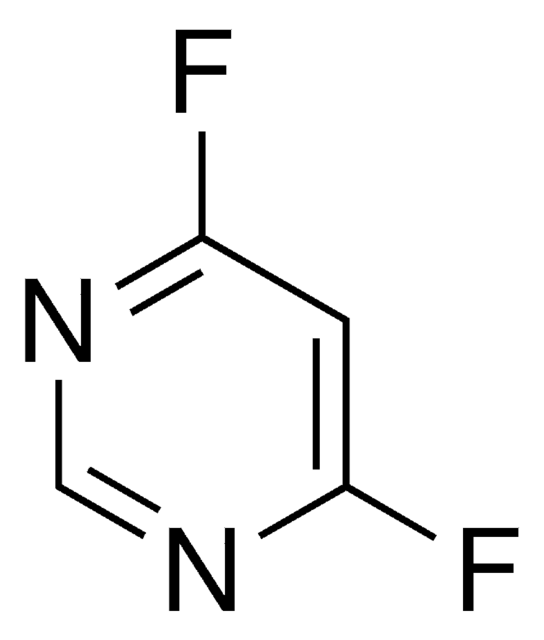
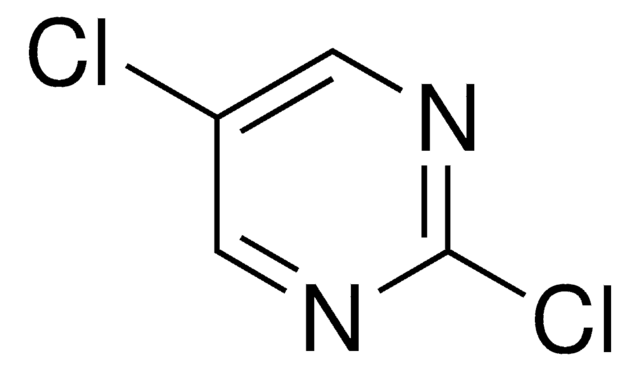
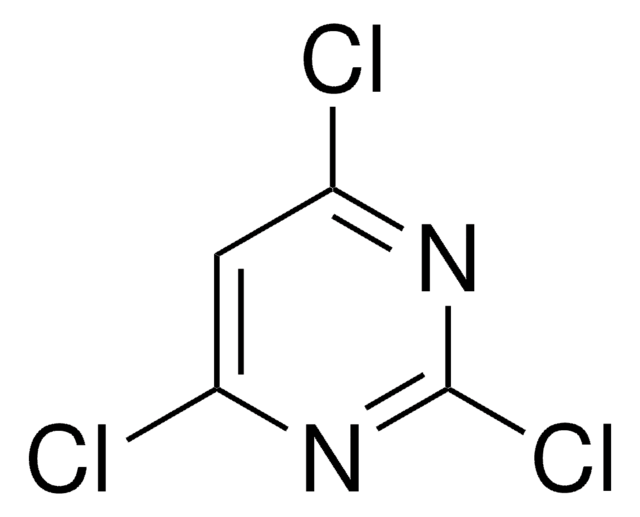
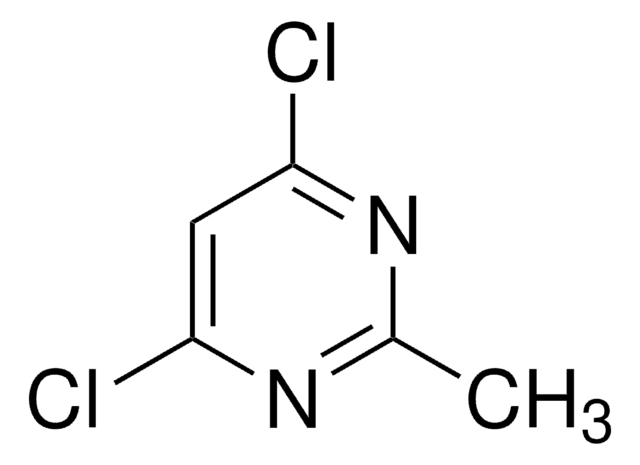
推荐产品
品質等級
化驗
97%
形狀
solid
bp
176 °C (lit.)
mp
65-67 °C (lit.)
溶解度
95% ethanol: soluble 50 mg/mL, clear to very slightly hazy, colorless to yellow
官能基
chloro
SMILES 字串
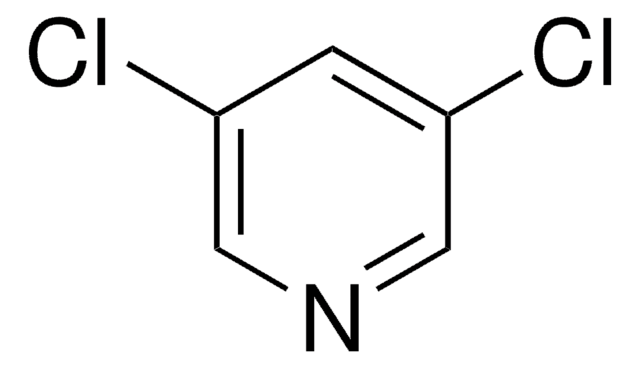
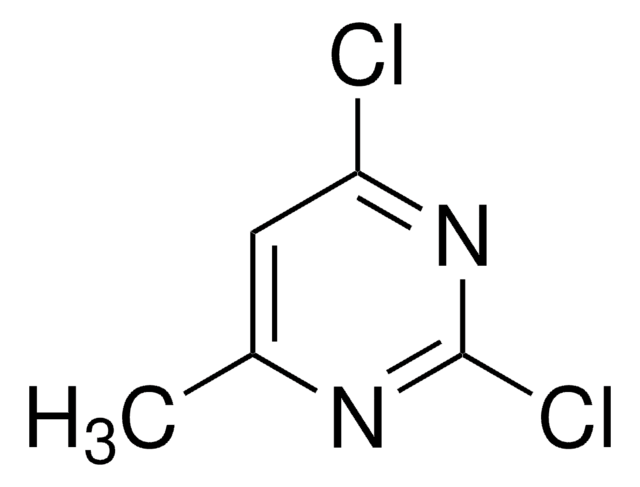
Clc1cc(Cl)ncn1
InChI
1S/C4H2Cl2N2/c5-3-1-4(6)8-2-7-3/h1-2H
InChI 密鑰
XJPZKYIHCLDXST-UHFFFAOYSA-N
正在寻找类似产品? 访问 产品对比指南
一般說明
4,6-二氯嘧啶的循环伏安图显示三个阴极波,由碳氯键的连续断裂以及嘧啶的还原产生 。
應用
在 N -取代氮杂杯嘧啶的合成中使用了 [4,6-二氯嘧啶 。它被用作通过串联胺化和 Suzuki-Miyaura 交叉偶联合成双取代嘧啶的起始试剂 。它也用于涉及联芳交叉耦合的联芳嘧啶合成。
訊號詞
Danger
危險聲明
危險分類
Skin Corr. 1B
儲存類別代碼
8A - Combustible corrosive hazardous materials
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
Eyeshields, Faceshields, Gloves, type P3 (EN 143) respirator cartridges
其他客户在看
Li-Xia Wang et al.
The Journal of organic chemistry, 75(3), 741-747 (2010-01-02)
A number of N-substituted azacalix[4]pyrimidines were synthesized by two methods. While straightforward condensation reaction between 4,6-dichloropyrimidine and 4,6-bis(alkylamino)pyrimidines gave identically N-substituted azacalix[4]pyrimidines in low yields, a general and moderate-to-high yielding 1 + 3 macrocyclic fragment coupling reaction afforded azacalix[4]pyrimidines that
Tetrahedron, 62, 10055-10055 (2006)
Electrochemical reduction of halogenated pyrimidines at mercury cathodes in acetonitrile.
Ji C, et al.
Journal of Electroanalytical Chemistry, 500(1), 3-11 (2001)
Richard T Wheelhouse et al.
Journal of medicinal chemistry, 49(17), 5187-5198 (2006-08-18)
Biarylpyrimidines are characterized as selective ligands for higher-order nucleic acid structures. A concise and efficient synthesis has been devised incorporating Suzuki biaryl cross-coupling of dihalopyrimidines. Two ligand series are described based on the parent thioether 4,6-bis[4-[[2-(dimethylamino)ethyl]mercapto]phenyl]pyrimidine (1a) and amide 4,6-bis(4[(2-(dimethylamino)ethyl)carboxamido]phenyl)pyrimidine
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门
![[1,1′-双(二苯基膦)二茂铁]二氯化钯(II)](/deepweb/assets/sigmaaldrich/product/structures/130/734/8846aa26-1858-458a-998d-8c306c13bf0f/640/8846aa26-1858-458a-998d-8c306c13bf0f.png)