B8959
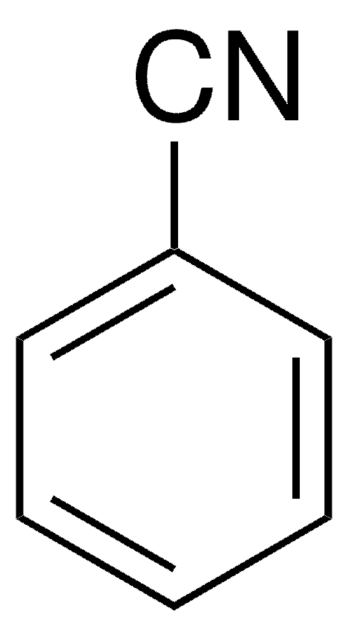
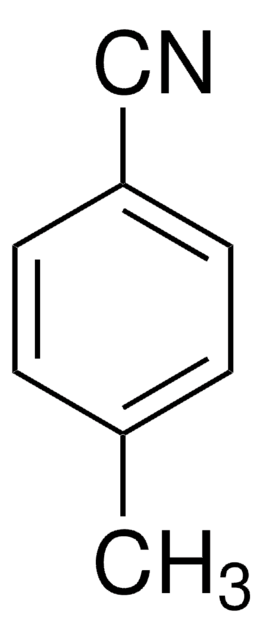
Benzonitrile
ReagentPlus®, 99%
Sinónimos:
Phenyl cyanide
About This Item
Productos recomendados
Nivel de calidad
Línea del producto
ReagentPlus®
Ensayo
99%
Formulario
liquid
lim. expl.
0.34-6.3 %
índice de refracción
n20/D 1.528 (lit.)
bp
191 °C (lit.)
mp
−13 °C (lit.)
cadena SMILES
N#Cc1ccccc1
InChI
1S/C7H5N/c8-6-7-4-2-1-3-5-7/h1-5H
Clave InChI
JFDZBHWFFUWGJE-UHFFFAOYSA-N
¿Está buscando productos similares? Visita Guía de comparación de productos
Descripción general
Aplicación
- An electrochemical solvent to investigate the electrochemistry, spectroscopic properties, and reactivity of a series of cobalt porphyrins with various substituents.
- Building block or starting material in various organic synthesis reactions.
- Employed in coupling reactions, such as Suzuki couplings or Heck reactions, to facilitate the formation of carbon-carbon bonds.
Información legal
Palabra de señalización
Warning
Frases de peligro
Consejos de prudencia
Clasificaciones de peligro
Acute Tox. 4 Dermal - Acute Tox. 4 Oral
Código de clase de almacenamiento
6.1A - Combustible acute toxic Cat. 1 and 2 / very toxic hazardous materials
Clase de riesgo para el agua (WGK)
WGK 1
Punto de inflamabilidad (°F)
158.0 °F - closed cup
Punto de inflamabilidad (°C)
70 °C - closed cup
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico