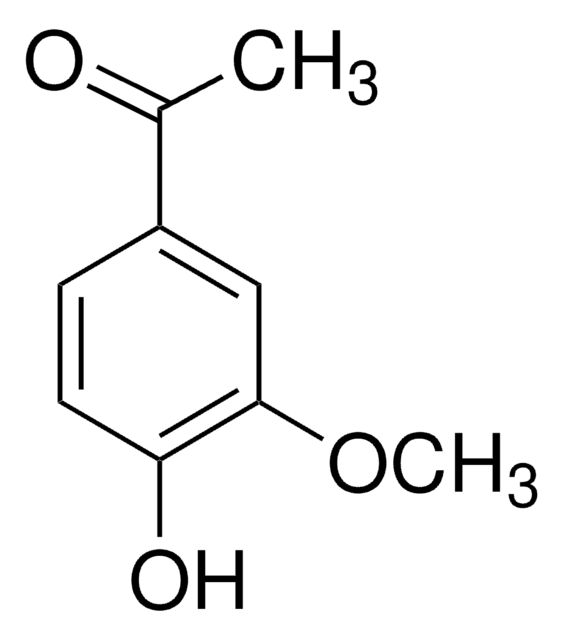
W404926
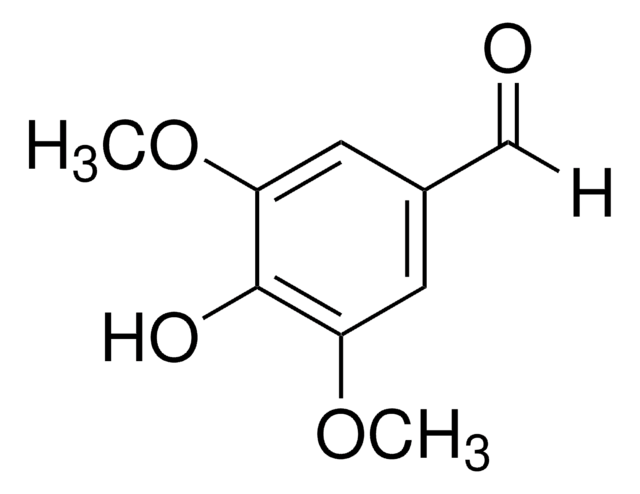
Syringaldehyde
≥98%, FG
Sinónimos:
3,5-Dimethoxy-4-hydroxybenzaldehyde, 4-Hydroxy-3,5-dimethoxybenzaldehyde
About This Item
Productos recomendados
origen biológico
synthetic
Nivel de calidad
grado
FG
cumplimiento norm.
EU Regulation 1334/2008 & 872/2012
FDA 21 CFR 110
Análisis
≥98%
bp
192-193 °C/14 mmHg (lit.)
mp
110-113 °C (lit.)
aplicaciones
flavors and fragrances
Documentación
see Safety & Documentation for available documents
alérgeno alimentario
no known allergens
Organoléptico
green; sweet
cadena SMILES
COc1cc(C=O)cc(OC)c1O
InChI
1S/C9H10O4/c1-12-7-3-6(5-10)4-8(13-2)9(7)11/h3-5,11H,1-2H3
Clave InChI
KCDXJAYRVLXPFO-UHFFFAOYSA-N
¿Está buscando productos similares? Visita Guía de comparación de productos
Aplicación
- Dual action of benzaldehydes: Inhibiting quorum sensing and enhancing antibiotic efficacy for controlling Pseudomonas aeruginosa biofilms.: This study investigates the dual action of syringaldehyde and other benzaldehydes in inhibiting quorum sensing and enhancing the efficacy of antibiotics against Pseudomonas aeruginosa biofilms, offering potential applications in antimicrobial therapies (Leitão et al., 2024).
- Development of Syringaldehyde as an Agonist of the GLP-1 Receptor to Alleviate Diabetic Disorders in Animal Models.: Research highlights the development of syringaldehyde as a novel agonist of the GLP-1 receptor, demonstrating significant potential in alleviating diabetic disorders in animal models (Lee et al., 2024).
- Aqueous-Phase Photoreactions of Mixed Aromatic Carbonyl Photosensitizers Yield More Oxygenated, Oxidized, and less Light-Absorbing Secondary Organic Aerosol (SOA) than Single Systems.: The study reveals that syringaldehyde, as part of mixed aromatic carbonyl photosensitizers, leads to the formation of highly oxygenated and oxidized secondary organic aerosols, impacting atmospheric chemistry and air quality (Mabato et al., 2024).
Acciones bioquímicas o fisiológicas
Otras notas
Palabra de señalización
Warning
Frases de peligro
Consejos de prudencia
Clasificaciones de peligro
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Órganos de actuación
Respiratory system
Código de clase de almacenamiento
11 - Combustible Solids
Clase de riesgo para el agua (WGK)
WGK 3
Equipo de protección personal
dust mask type N95 (US), Eyeshields, Gloves
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico