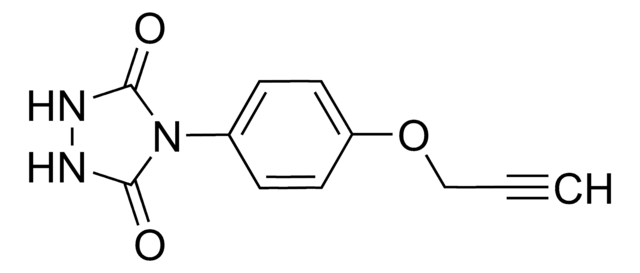
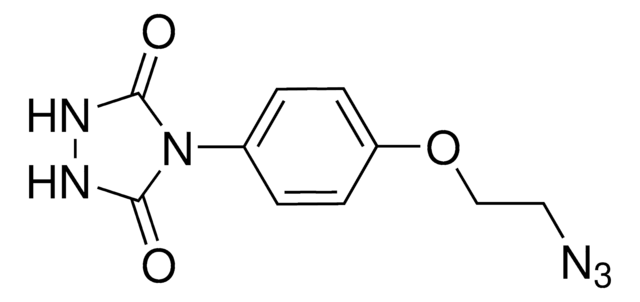
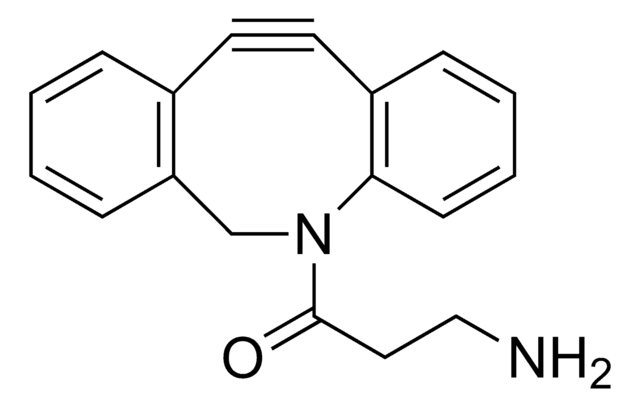
ALD00344
PTAD-Azide
95%
Sinónimos:
4-(4-(2-Azidoethoxy)phenyl)-1,2,4-triazolidine-3,5-dione, N3-Ph-Ur for e-Y-CLICK
About This Item
Productos recomendados
Nivel de calidad
Ensayo
95%
Formulario
powder or crystals
idoneidad de la reacción
reagent type: cross-linking reagent
grupo funcional
azide
temp. de almacenamiento
2-8°C
cadena SMILES
O=C(NNC1=O)N1C2=CC=C(OCCN=[N+]=[N-])C=C2
InChI
1S/C10H10N6O3/c11-15-12-5-6-19-8-3-1-7(2-4-8)16-9(17)13-14-10(16)18/h1-4H,5-6H2,(H,13,17)(H,14,18)
Clave InChI
MHGMHPVYCVQIET-UHFFFAOYSA-N
¿Está buscando productos similares? Visita Guía de comparación de productos
Descripción general
Aplicación
Note: PTAD-Azide must be first activated by stirring in a 1:0.98 molar ratio with 1,3-dibromo-5,5-dimethylhydantoin (product # 157902). Activation is evident upon solution color change from colorless to deep red and the activated reagent should be used immediately.
General Procedure for Protein Modification with PTAD.
Part 1: PTAD activation
- Mix together 1:0.98 molar equivalents of unactivated PTAD to 1,3-dibromo-5,5-dimethylhydantoin (product # 157902) in organic solvent (preferred solvents are DMF or acetonitrile, avoid using DMSO)
- Color change is observed from colorless/pale yellow to deep red (approximately 5 min of mixing).
- After the solution turns red, store the now activated reagent on ice and use for protein modification within 30 min.
Part 2: Protein modification
- Add protein solution in mixed phosphate/Tris buffer or Tris buffer (pH should be 6 - 9) to the eppendorf tube (or other vial) containing the activated PTAD reagent prepared above and mix gently at room temperature for up to 30 min. Preferably use 10-fold molar excess of reagent relative to protein. Use protein at a minimum concentration of 1 mg/ml (higher concentrations are preferred for enhanced labeling).
- Remove excess unreacted PTAD by gel filtration.
Producto relacionado
Código de clase de almacenamiento
11 - Combustible Solids
Clase de riesgo para el agua (WGK)
WGK 1
Punto de inflamabilidad (°F)
Not applicable
Punto de inflamabilidad (°C)
Not applicable
Elija entre una de las versiones más recientes:
Certificados de análisis (COA)
¿No ve la versión correcta?
Si necesita una versión concreta, puede buscar un certificado específico por el número de lote.
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico