280992
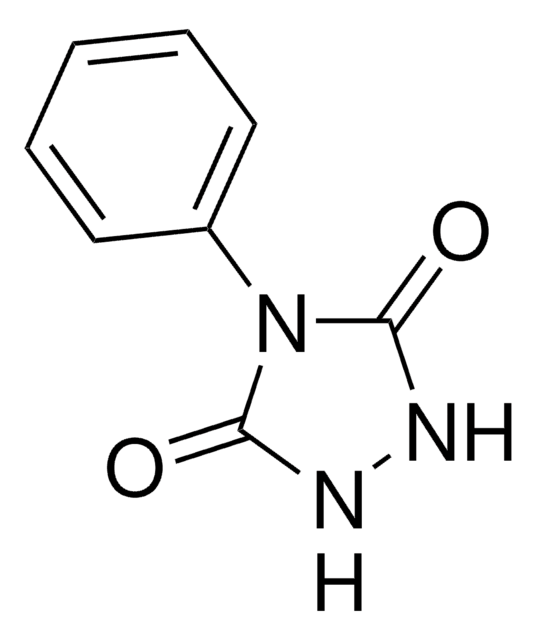
4-Phenyl-1,2,4-triazoline-3,5-dione
97%
Sinónimos:
4-Phenyl-3H-1,2,4-triazole-3,5(4H)-dione, PTAD
Iniciar sesiónpara Ver la Fijación de precios por contrato y de la organización
About This Item
Fórmula empírica (notación de Hill):
C8H5N3O2
Número de CAS:
Peso molecular:
175.14
Beilstein:
141548
Número CE:
Número MDL:
Código UNSPSC:
12352005
ID de la sustancia en PubChem:
NACRES:
NA.22
Productos recomendados
Nivel de calidad
Ensayo
97%
Formulario
solid
mp
165-170 °C (dec.) (lit.)
temp. de almacenamiento
2-8°C
cadena SMILES
O=C1N=NC(=O)N1c2ccccc2
InChI
1S/C8H5N3O2/c12-7-9-10-8(13)11(7)6-4-2-1-3-5-6/h1-5H
Clave InChI
ISULLEUFOQSBGY-UHFFFAOYSA-N
¿Está buscando productos similares? Visita Guía de comparación de productos
Categorías relacionadas
Aplicación
4-Phenyl-1,2,4-triazoline-3,5-dione (PTAD) can be used as an efficient and selective reagent for the oxidation of thiols to disulfides.
It can also be used:
It can also be used:
- As a dehydrogenating agent to synthesize annulated dihydropyridazines by inverse [4+2] cycloaddition reaction between cyclic alkenes and 1,2,4,5-tetrazines.
- As a dienophile to synthesize cycloaddition products by fast hetero-Diels−Alder reactions.
- As an efficient oxidizing agent for the synthesis of pyridine derivatives from 1,4-dihydropyridines.
- In the synthesis of urazoles via [3+2] cycloaddition with allylsilanes.
Código de clase de almacenamiento
11 - Combustible Solids
Clase de riesgo para el agua (WGK)
WGK 3
Punto de inflamabilidad (°F)
Not applicable
Punto de inflamabilidad (°C)
Not applicable
Equipo de protección personal
Eyeshields, Gloves, type N95 (US)
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Location of conjugated diene position in an aliphatic chain by mass spectrometry of the 4-phenyl-1,2,4-triazoline-3,5-dione adduct.
D C Young et al.
Analytical chemistry, 59(15), 1954-1957 (1987-08-01)
Tatsuya Higashi et al.
Analytical and bioanalytical chemistry, 403(2), 495-502 (2012-03-01)
The utility of Diels-Alder derivatization with 4-phenyl-1,2,4-triazoline-3,5-dione (PTAD) for liquid chromatography/electrospray ionization tandem mass spectrometry of conjugated linoleic acids (CLAs) was examined. PTAD rapidly reacted with the CLAs, and the resulting derivatives were highly responsive in electrospray ionization mass spectrometry
Tetrahedron Letters, 48, 6671-6671 (2007)
4-Phenyl-1, 2, 4-triazole-3, 5-dione as a novel and reusable reagent for the aromatization of 1, 4-dihydropyridines under mild conditions
Zolfigol MA, et al.
Tetrahedron Letters, 46(33), 5581-5584 (2005)
K Søndergaard et al.
Chemistry (Weinheim an der Bergstrasse, Germany), 7(11), 2324-2331 (2001-07-12)
The 5-aza-6-deoxy analogue of castanospermine (+/-)-5a and its 1-epimer (+/-)-5b was synthesized. The synthesis started from the known compound 5-benzyloxy-7-hydroxyhepta-1,3-diene, which was protected and subjected to Diels-Alder reaction with 4-phenyl-1,2,4-triazoline-3,5-dione to give two epimeric adducts. One of these was transformed
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico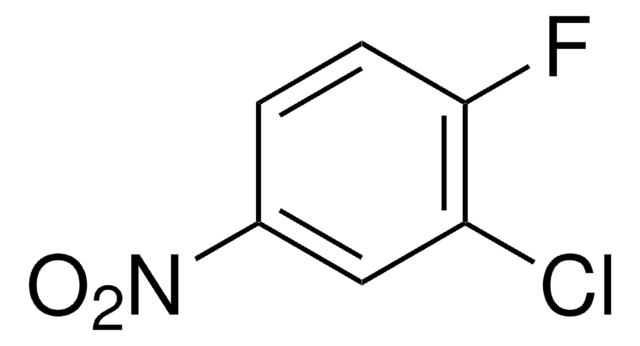



![1-clorometil-4-fluoro-1,4-diazoniabiciclo[2,2.2]octano bis(tetrafluoroborato) >95% in F+ active](/deepweb/assets/sigmaaldrich/product/structures/206/487/53d52ee5-ef71-4e9a-9bc8-938b68b98d5d/640/53d52ee5-ef71-4e9a-9bc8-938b68b98d5d.png)

