Wichtige Dokumente
M5793
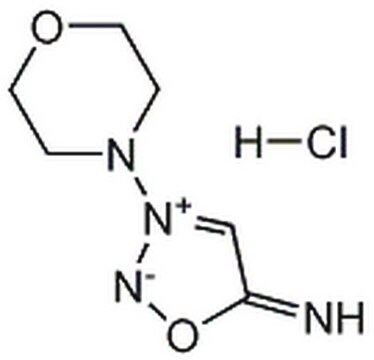
3-Morpholinosydnonimine hydrochloride
(consistent with structure, NMR)
Synonym(e):
3-(4-Morpholinyl)sydnone imine hydrochloride, Linsidomine hydrochloride, SIN-1 hydrochloride
About This Item
Empfohlene Produkte
Qualitätsniveau
Lagertemp.
−20°C
SMILES String
Cl[H].[NH-]c1c[n+](no1)N2CCOCC2
InChI
1S/C6H10N4O2.ClH/c7-6-5-10(8-12-6)9-1-3-11-4-2-9;/h5,7H,1-4H2;1H
InChIKey
NCGICGYLBXGBGN-UHFFFAOYSA-N
Suchen Sie nach ähnlichen Produkten? Aufrufen Leitfaden zum Produktvergleich
Verwandte Kategorien
Anwendung
- as a peroxynitrite donor standard to study the accumulation of peroxynitrite in Arabidopsis thaliana
- to study its effects on nitrosative stress in human brain vascular pericytes and human embryonic kidney cells
- as a ROS to study its effects on mouse embryonic fibroblasts
Biochem./physiol. Wirkung
Lagerklassenschlüssel
11 - Combustible Solids
WGK
WGK 2
Flammpunkt (°F)
Not applicable
Flammpunkt (°C)
Not applicable
Persönliche Schutzausrüstung
Eyeshields, Gloves, type N95 (US)
Hier finden Sie alle aktuellen Versionen:
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
Kunden haben sich ebenfalls angesehen
Artikel
Nitric oxide (NO) as a signal transporter in neurons, endothelial cells and in the immune system.
Verwandter Inhalt
DISCOVER Bioactive Small Molecules for Nitric Oxide & Cell Stress Research
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.