E5389
Erythromycin
powder, suitable for cell culture, BioReagent
Synonym(e):
E-Mycin, Erythrocin
About This Item
Empfohlene Produkte
product name
Erythromycin, BioReagent, suitable for cell culture
Produktlinie
BioReagent
Form
powder
Wirksamkeit
≥850 μg per mg
Methode(n)
cell culture | mammalian: suitable
Verunreinigungen
≤0.1 EU/mg endotoxin
Farbe
white
mp (Schmelzpunkt)
133 °C
Löslichkeit
2 M HCl: 50 mg/mL (Stock solutions should be stored at 2-8 °C. Stable at 37 °C for 3 days.)
ethanol: soluble (Stock solutions should be stored at 2-8 °C. Stable at 37 °C for 3 days.)
Wirkungsspektrum von Antibiotika
Gram-negative bacteria
Gram-positive bacteria
Wirkungsweise
protein synthesis | interferes
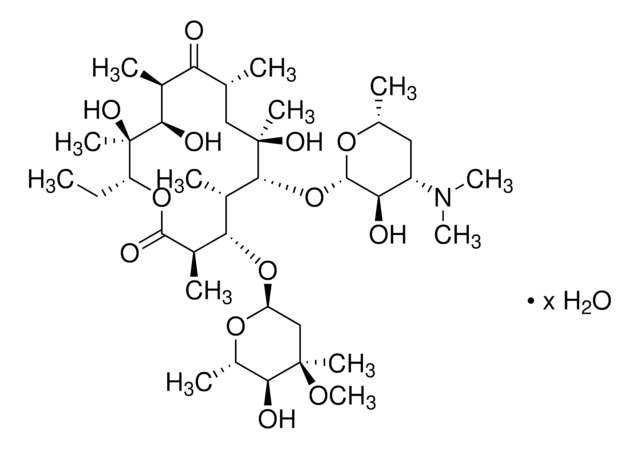
SMILES String
CC[C@H]1OC(=O)[C@H](C)[C@@H](O[C@H]2C[C@@](C)(OC)[C@@H](O)[C@H](C)O2)[C@H](C)[C@@H](O[C@@H]3O[C@H](C)C[C@@H]([C@H]3O)N(C)C)[C@](C)(O)C[C@@H](C)C(=O)[C@H](C)[C@@H](O)[C@]1(C)O
InChI
1S/C37H67NO13/c1-14-25-37(10,45)30(41)20(4)27(39)18(2)16-35(8,44)32(51-34-28(40)24(38(11)12)15-19(3)47-34)21(5)29(22(6)33(43)49-25)50-26-17-36(9,46-13)31(42)23(7)48-26/h18-26,28-32,34,40-42,44-45H,14-17H2,1-13H3/t18-,19-,20+,21+,22-,23+,24+,25-,26+,28-,29+,30-,31+,32-,34+,35-,36-,37-/m1/s1
InChIKey
ULGZDMOVFRHVEP-RWJQBGPGSA-N
Angaben zum Gen
human ... ABCB1(5243) , CYP3A4(1576) , MLNR(2862)
mouse ... Abcb1a(18671) , Abcb1b(18669)
Suchen Sie nach ähnlichen Produkten? Aufrufen Leitfaden zum Produktvergleich
Allgemeine Beschreibung
Anwendung
- as a supplement for nutrient broth medium for culturing green fluorescent protein (GFP)- expressing E. coli
- as a model drug to determine small intestinal (SMI) microtissue viability using the MTT assay{254
- as an antibiotic to study the treatment strategies of chronic infections
Biochem./physiol. Wirkung
Antimikrobielles Spektrum: Gram-negative und Gram-positive Bakterien.
Vorsicht
Angaben zur Herstellung
Lagerklassenschlüssel
11 - Combustible Solids
WGK
WGK 3
Flammpunkt (°F)
Not applicable
Flammpunkt (°C)
Not applicable
Persönliche Schutzausrüstung
Eyeshields, Gloves, type N95 (US)
Analysenzertifikate (COA)
Suchen Sie nach Analysenzertifikate (COA), indem Sie die Lot-/Chargennummer des Produkts eingeben. Lot- und Chargennummern sind auf dem Produktetikett hinter den Wörtern ‘Lot’ oder ‘Batch’ (Lot oder Charge) zu finden.
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
Kunden haben sich ebenfalls angesehen
Artikel
Protein synthesis is a complex, multi-step process involving many enzymes as well as conformational alignment. However, the majority of antibiotics that block bacterial protein synthesis interfere with the processes at the 30S subunit or 50S subunit of the 70S bacterial ribosome.
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.