1066009
USP
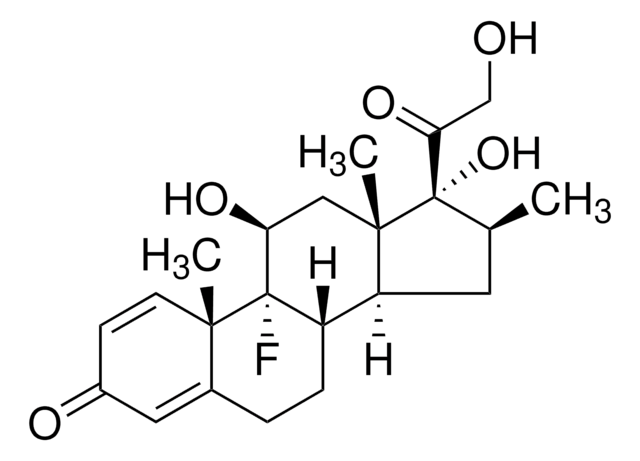
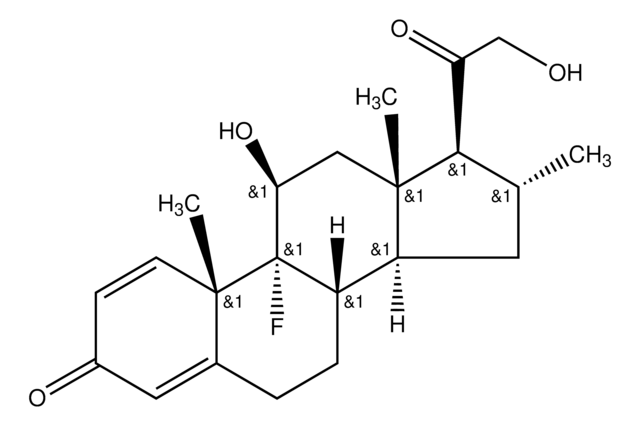
Betamethasone
United States Pharmacopeia (USP) Reference Standard
Sinonimo/i:
9α-Fluoro-11β,17α,21-trihydroxy-16β-methylpregna-1,4-diene-3,20-dione, 9α-Fluoro-16β-methyl-11β,17α,21-trihydroxy-1,4-pregnadiene-3,20-dione, 9α-Fluoro-16β-methylprednisolone
About This Item
Prodotti consigliati
Origine biologica
synthetic
Grado
pharmaceutical primary standard
agenzia
USP
Tensione di vapore
<0.0000001 kPa ( 25 °C)
Famiglia di API
betamethasone
Confezionamento
pkg of 200 mg
Produttore/marchio commerciale
USP
Colore
white to off-white
Punto di fusione
235-237 °C (lit.)
447.8-473 °F (231—245°C; decomposes)
Solubilità
acetone: sparingly soluble
chloroform: very slightly soluble
ethanol: sparingly soluble
ether: very slightly soluble
methanol: sparingly soluble
water: insoluble
Densità
0.305 g/cm3 at 25 °C (77°F)
applicazioni
pharmaceutical (small molecule)
Formato
neat
Temperatura di conservazione
2-8°C
Stringa SMILE
[H][C@@]12C[C@H](C)[C@](O)(C(=O)CO)[C@@]1(C)C[C@H](O)[C@@]3(F)[C@@]2([H])CCC4=CC(=O)C=C[C@]34C
InChI
1S/C22H29FO5/c1-12-8-16-15-5-4-13-9-14(25)6-7-19(13,2)21(15,23)17(26)10-20(16,3)22(12,28)18(27)11-24/h6-7,9,12,15-17,24,26,28H,4-5,8,10-11H2,1-3H3/t12-,15-,16-,17-,19-,20-,21-,22-/m0/s1
UREBDLICKHMUKA-DVTGEIKXSA-N
Informazioni sul gene
human ... NR3C1(2908)
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Descrizione generale
This product is provided as delivered and specified by the issuing Pharmacopoeia. All information provided in support of this product, including SDS and any product information leaflets have been developed and issued under the Authority of the issuing Pharmacopoeia.For further information and support please go to the website of the issuing Pharmacopoeia.
Applicazioni
- Betamethasone Acetate
- Betamethasone Oral Solution
- Betamethasone Valerate Cream
- Betamethasone Valerate Lotion
- Betamethasone Valerate Ointment
- Dexamethasone
Risultati analitici
Altre note
Prodotti correlati
Avvertenze
Danger
Indicazioni di pericolo
Consigli di prudenza
Classi di pericolo
Repr. 1B - STOT RE 2
Organi bersaglio
Liver,Kidney,Endocrine system
Codice della classe di stoccaggio
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
Classe di pericolosità dell'acqua (WGK)
WGK 2
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Scegli una delle versioni più recenti:
Certificati d'analisi (COA)
Ci dispiace, ma al momento non ci sono COA disponibili online per questo prodotto.
Se ti serve aiuto, non esitare a contattarci Servizio Clienti
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
Protocolli
A simple, precise and sensitive Reverse-Phase High Pressure Liquid Chromatography gradient method was adapted for traceability, homogeneity and total chromatographic analysis of Dexamethasone. The given experimental conditions follow the USP43-NF38 monograph method for Dexamethasone Assay and Organic Impurity Profiling. Dexamethasone, Betamethasone, Dexamethasone acetate and Desoximetasone were baseline resolved within 20 minutes using a Titan C18 UHPLC column (2.1 x 100 mm, 1.9 µm).
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.