PHR1398
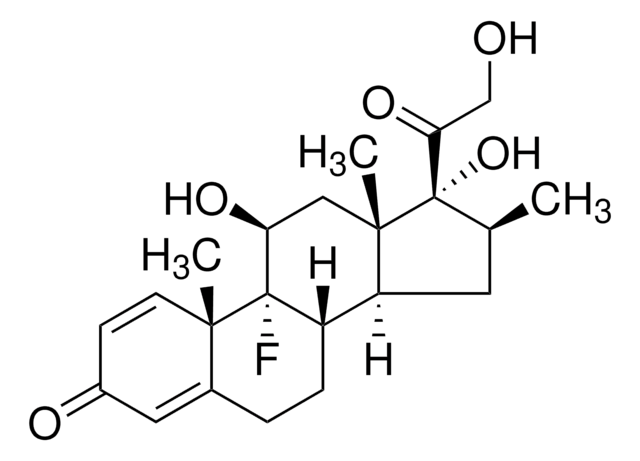
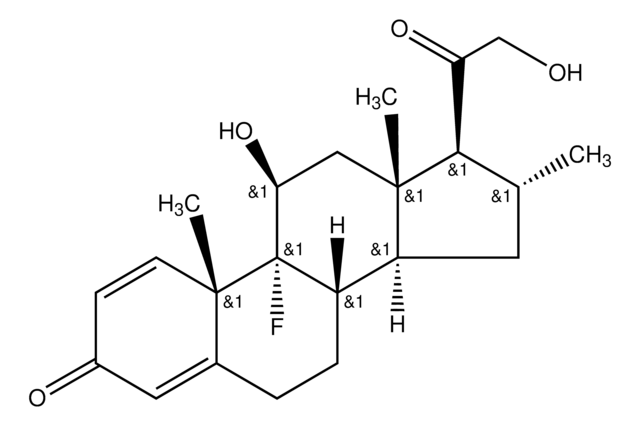
Betamethasone
Pharmaceutical Secondary Standard; Certified Reference Material
Sinonimo/i:
Betamethasone, 9α-Fluoro-11β,17α,21-trihydroxy-16β-methylpregna-1,4-diene-3,20-dione, 9α-Fluoro-16β-methyl-11β,17α,21-trihydroxy-1,4-pregnadiene-3,20-dione, 9α-Fluoro-16β-methylprednisolone
About This Item
Prodotti consigliati
Origine biologica
synthetic
Livello qualitativo
Grado
certified reference material
pharmaceutical secondary standard
agenzia
BP
EP
USP
traceable to BP 575
traceable to Ph. Eur. B1000000
traceable to USP 1066009
Tensione di vapore
<0.0000001 kPa ( 25 °C)
Famiglia di API
betamethasone
CdA
current certificate can be downloaded
Confezionamento
pkg of 1 g
Condizioni di stoccaggio
protect from light (20 mm aluminium crimp seal for unused portion)
tecniche
HPLC: suitable
gas chromatography (GC): suitable
Colore
white to light yellow
Punto di fusione
235-237 °C (lit.)
Solubilità
acetone: sparingly soluble
chloroform: very slightly soluble
ethanol: sparingly soluble
ether: very slightly soluble
methanol: sparingly soluble
water: insoluble
Densità
0.305 g/cm3 at 25 °C (77°F)
applicazioni
pharmaceutical (small molecule)
Formato
neat
Condizioni di spedizione
ambient
Temperatura di conservazione
2-30°C
Stringa SMILE
[H][C@@]12C[C@H](C)[C@](O)(C(=O)CO)[C@@]1(C)C[C@H](O)[C@@]3(F)[C@@]2([H])CCC4=CC(=O)C=C[C@]34C
InChI
1S/C22H29FO5/c1-12-8-16-15-5-4-13-9-14(25)6-7-19(13,2)21(15,23)17(26)10-20(16,3)22(12,28)18(27)11-24/h6-7,9,12,15-17,24,26,28H,4-5,8,10-11H2,1-3H3/t12-,15-,16-,17-,19-,20-,21-,22-/m0/s1
UREBDLICKHMUKA-DVTGEIKXSA-N
Informazioni sul gene
human ... NR3C1(2908)
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Descrizione generale
Betamethasone belongs to the corticosteriod family of active pharmaceutical ingredients (APIs). It exhibits anti-inflammatory activity and hence is used in the manufacture of various finished pharmaceutical products and is also employed as a starting material to manufacture other APIs that are related to this steroid family.
Applicazioni
Risultati analitici
Altre note
Nota a piè di pagina
Prodotti correlati
Avvertenze
Danger
Indicazioni di pericolo
Consigli di prudenza
Classi di pericolo
Repr. 1B - STOT RE 2
Organi bersaglio
Liver,Kidney,Endocrine system
Codice della classe di stoccaggio
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
Classe di pericolosità dell'acqua (WGK)
WGK 2
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.