S3626
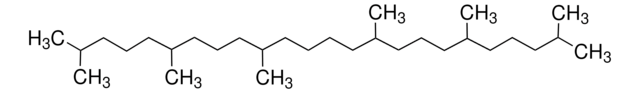
Squalene
≥98%, liquid
Sinonimo/i:
2,6,10,15,19,23-Hexamethyl-2,6,10,14,18,22-tetracosahexaene
About This Item
Prodotti consigliati
Saggio
≥98%
Stato
liquid
Colore
light yellow
Indice di rifrazione
n20/D 1.494 (lit.)
P. ebollizione
285 °C/25 mmHg (lit.)
Punto di fusione
−75 °C (lit.)
Densità
0.858 g/mL at 25 °C (lit.)
applicazioni
metabolomics
vitamins, nutraceuticals, and natural products
Temperatura di conservazione
2-8°C
Stringa SMILE
CC(C)=CCCC(C)=CCCC(C)=CCC\C=C(/C)CCC=C(C)CCC=C(C)C
InChI
1S/C30H50/c1-25(2)15-11-19-29(7)23-13-21-27(5)17-9-10-18-28(6)22-14-24-30(8)20-12-16-26(3)4/h15-18,23-24H,9-14,19-22H2,1-8H3/b27-17+,28-18+,29-23+,30-24+
YYGNTYWPHWGJRM-AAJYLUCBSA-N
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Descrizione generale
Applicazioni
- as a standard for lipid identification and quantification
- in the isolation of macrophages for parasite incubation
- as a standard for the quantification of squalene in squalene analysis of oil samples
Azioni biochim/fisiol
Avvertenze
Danger
Indicazioni di pericolo
Consigli di prudenza
Classi di pericolo
Asp. Tox. 1
Codice della classe di stoccaggio
10 - Combustible liquids
Classe di pericolosità dell'acqua (WGK)
WGK 2
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Dispositivi di protezione individuale
Eyeshields, Gloves
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
Articoli
Biosynthesis of cholesterol generally takes place in the endoplasmic reticulum of hepatic cells and begins with acetyl- CoA, which is mainly derived from an oxidation reaction in the mitochondria. Acetyl-CoA and acetoacetyl-CoA are converted to 3-hydroxy- 3-methylglutaryl-CoA (HMG-CoA) by HMG-CoA synthase.
Dietary Terpenes
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.