M6626
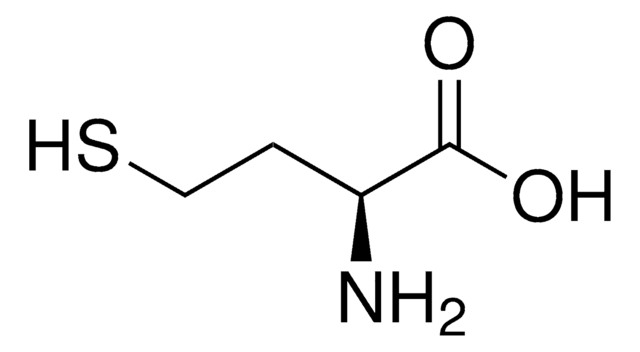
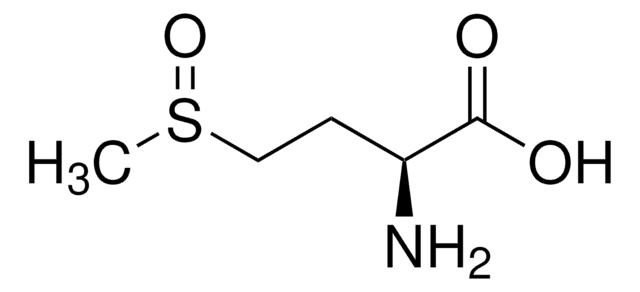
S-Methyl-L-cysteine
suitable for ligand binding assays
Sinonimo/i:
(R)-2-Amino-3-(methylmercapto)propionic acid, SMLC
About This Item
Prodotti consigliati
product name
S-Methyl-L-cysteine, substrate for methionine sulfoxide reductase A
Livello qualitativo
Forma fisica
powder
tecniche
ligand binding assay: suitable
applicazioni
peptide synthesis
Temperatura di conservazione
−20°C
Stringa SMILE
CSC[C@H](N)C(O)=O
InChI
1S/C4H9NO2S/c1-8-2-3(5)4(6)7/h3H,2,5H2,1H3,(H,6,7)/t3-/m0/s1
IDIDJDIHTAOVLG-VKHMYHEASA-N
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Applicazioni
- In-vitro antioxidant and anti-inflammatory potential along with p.o. pharmacokinetic profile of key bioactive phytocompounds of Snow Mountain Garlic: a comparative analysis vis-à-vis normal garlic.: This study examines the antioxidant and anti-inflammatory properties of S-Methyl-L-cysteine found in Snow Mountain Garlic, providing insights into its potential therapeutic applications (Kaur et al., 2024).
- Acidification and tissue disruption affect glucosinolate and S-methyl-l-cysteine sulfoxide hydrolysis and formation of amines, isothiocyanates and other organosulfur compounds in red cabbage (Brassica oleracea var. capitata f. rubra).: The research highlights how acidification and tissue disruption influence the hydrolysis of S-Methyl-L-cysteine sulfoxide, impacting the formation of various bioactive compounds in red cabbage (Hanschen, 2024).
- Selective cycloaddition of ethylene oxide to CO(2) within the confined space of an amino acid-based metal-organic framework.: This research explores the use of an S-Methyl-L-cysteine-based metal-organic framework for the selective cycloaddition of ethylene oxide to CO2, demonstrating its potential in green chemistry and materials science (Bilanin et al., 2023).
Azioni biochim/fisiol
When used as a dietary supplement in the Drosphilia model of PD, SMLC increases the efficacy of the MRSA catalytic antioxidant system by providing additional substrate available leading to increased resistance to oxidative stress.
Codice della classe di stoccaggio
11 - Combustible Solids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Dispositivi di protezione individuale
Eyeshields, Gloves, type N95 (US)
Certificati d'analisi (COA)
Cerca il Certificati d'analisi (COA) digitando il numero di lotto/batch corrispondente. I numeri di lotto o di batch sono stampati sull'etichetta dei prodotti dopo la parola ‘Lotto’ o ‘Batch’.
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.