K1876
Kanamycin disulfate salt from Streptomyces kanamyceticus
aminoglycoside antibiotic
Sinonimo/i:
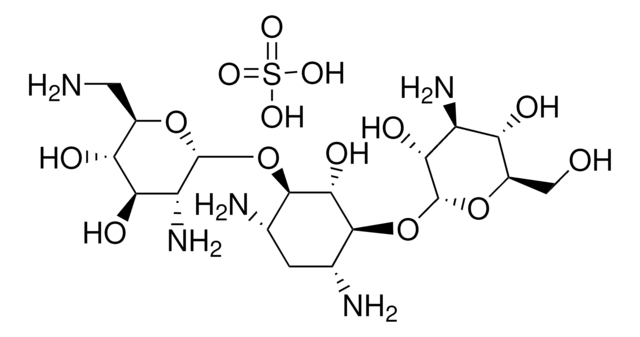
Kanamycin Disulfate Salt, Kanamycin disulfate, O-3-amino-3-deoxy-alpha-D-glucopyranosyl-(1->6)-O-(6-amino-6-deoxy-alpha-D-glucopyranosyl-(1->4))-2-deoxy-D-Streptamine sulfate (1:2) (salt), Kanamycin A, Kanamycin acid sulfate
About This Item
Prodotti consigliati
Origine biologica
Streptomyces kanamyceticus
Livello qualitativo
Forma fisica
powder
Potenza
~650 μg per mg
Impurezze
≤4% Kanamycin B
Colore
white to off-white
Solubilità
H2O: 10 mg/mL
Spettro attività antibiotica
Gram-negative bacteria
Gram-positive bacteria
mycobacteria
mycoplasma
Modalità d’azione
protein synthesis | interferes
Stringa SMILE
OS(O)(=O)=O.OS(O)(=O)=O.NC[C@H]1O[C@H](O[C@@H]2[C@@H](N)C[C@@H](N)[C@H](O[C@H]3O[C@H](CO)[C@@H](O)[C@H](N)[C@H]3O)[C@H]2O)[C@H](O)[C@@H](O)[C@@H]1O
InChI
1S/C18H36N4O11.2H2O4S/c19-2-6-10(25)12(27)13(28)18(30-6)33-16-5(21)1-4(20)15(14(16)29)32-17-11(26)8(22)9(24)7(3-23)31-17;2*1-5(2,3)4/h4-18,23-29H,1-3,19-22H2;2*(H2,1,2,3,4)/t4-,5+,6-,7-,8+,9-,10-,11-,12+,13-,14-,15+,16-,17-,18-;;/m1../s1
OGTKIXVMLDAMNU-KNQICTBBSA-N
Descrizione generale
Applicazioni
Azioni biochim/fisiol
Mode of Resistance: Aminoglycoside-modifying enzymes (including acetyltransferase, phosphotransferase, nucleotidyltransferase) can alter this antibiotic, preventing its interaction with ribosomes.
Antimicrobial Spectrum: Kanamycin sulfate is effective against gram-negative and gram-postiive bacteria, and mycoplasma.
Avvertenza
Nota sulla preparazione
Avvertenze
Danger
Indicazioni di pericolo
Consigli di prudenza
Classi di pericolo
Repr. 1B
Codice della classe di stoccaggio
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Dispositivi di protezione individuale
Eyeshields, Gloves, type P3 (EN 143) respirator cartridges
Certificati d'analisi (COA)
Cerca il Certificati d'analisi (COA) digitando il numero di lotto/batch corrispondente. I numeri di lotto o di batch sono stampati sull'etichetta dei prodotti dopo la parola ‘Lotto’ o ‘Batch’.
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
Articoli
Protein synthesis is a complex, multi-step process involving many enzymes as well as conformational alignment. However, the majority of antibiotics that block bacterial protein synthesis interfere with the processes at the 30S subunit or 50S subunit of the 70S bacterial ribosome.
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.