A8717
Anti-Amyloid Precursor Protein (APP) Antibody

rabbit polyclonal
Sinonimo/i:
APP antibody, Anti-APP
About This Item
Prodotti consigliati
Nome del prodotto
Anti-Amyloid Precursor Protein, C-Terminal antibody produced in rabbit, IgG fraction of antiserum, buffered aqueous solution
Origine biologica
rabbit
Livello qualitativo
Coniugato
unconjugated
Forma dell’anticorpo
IgG fraction of antiserum
Tipo di anticorpo
primary antibodies
Clone
polyclonal
Stato
buffered aqueous solution
PM
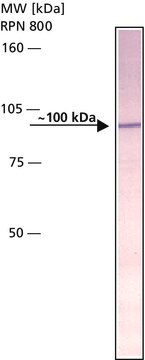
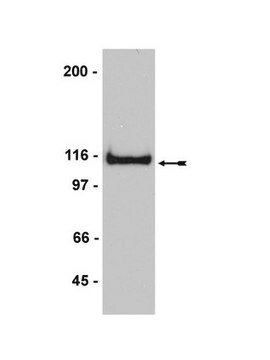
antigen 95-100 kDa
Reattività contro le specie
mouse, human, rat
Confezionamento
antibody small pack of 25 μL
Convalida avanzata
recombinant expression
Learn more about Antibody Enhanced Validation
tecniche
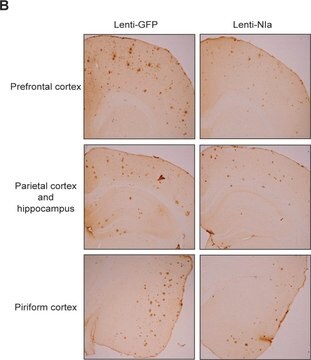
immunohistochemistry (formalin-fixed, paraffin-embedded sections): 1:1000 using formic acid-treated sections of human Alzheimer′s disease (AD) brain
microarray: suitable
western blot: 1:4,000 using rat brain extract
N° accesso UniProt
Condizioni di spedizione
dry ice
Temperatura di conservazione
−20°C
modifica post-traduzionali bersaglio
unmodified
Informazioni sul gene
human ... APP(351)
mouse ... App(11820)
rat ... App(54226)
Descrizione generale
Immunogeno
Applicazioni
Azioni biochim/fisiol
Stato fisico
Stoccaggio e stabilità
Esclusione di responsabilità
Non trovi il prodotto giusto?
Prova il nostro Motore di ricerca dei prodotti.
Raccomandato
Codice della classe di stoccaggio
12 - Non Combustible Liquids
Classe di pericolosità dell'acqua (WGK)
nwg
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.