10810
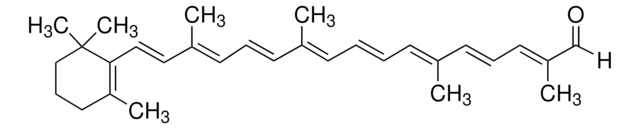
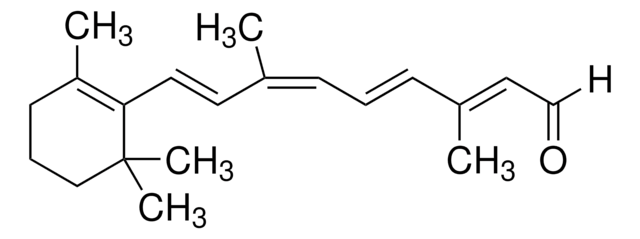
trans-β-Apo-8′-carotenal
≥96.0% (UV)
Sinonimo/i:
Apocarotenal
About This Item
Prodotti consigliati
Origine biologica
synthetic
Livello qualitativo
Saggio
≥96.0% (UV)
Stato
powder
Perdita
≤0.5% loss on drying, 20 °C (HV)
Punto di fusione
137-141 °C
Solubilità
chloroform: 1 mg/mL, clear to very faintly turbid, intense red-orange
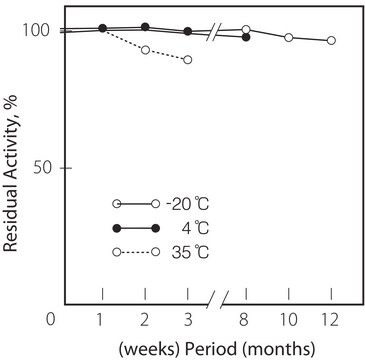
Temperatura di conservazione
−20°C
Stringa SMILE
[H]C(=O)\C(C)=C\C=C\C(C)=C\C=C\C=C(C)\C=C\C=C(C)\C=C\C1=C(C)CCCC1(C)C
InChI
1S/C30H40O/c1-24(13-8-9-14-25(2)16-11-18-27(4)23-31)15-10-17-26(3)20-21-29-28(5)19-12-22-30(29,6)7/h8-11,13-18,20-21,23H,12,19,22H2,1-7H3/b9-8+,15-10+,16-11+,21-20+,24-13+,25-14+,26-17+,27-18+
DFMMVLFMMAQXHZ-DOKBYWHISA-N
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Applicazioni
- Tackling the challenge of selective analytical clean-up of complex natural extracts: the curious case of chlorophyll removal: This study outlines a methodology for the selective analytical clean-up of complex natural extracts, which can be applied to enhance the purity and stability of trans-β-Apo-8′-carotenal, particularly useful in the context of life science manufacturing and research and development. This approach is crucial for maintaining the integrity of bioactive compounds during synthesis and storage (Bijttebier et al., 2014).
Azioni biochim/fisiol
Altre note
Codice della classe di stoccaggio
11 - Combustible Solids
Classe di pericolosità dell'acqua (WGK)
WGK 2
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Dispositivi di protezione individuale
Eyeshields, Gloves, type N95 (US)
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.