86320
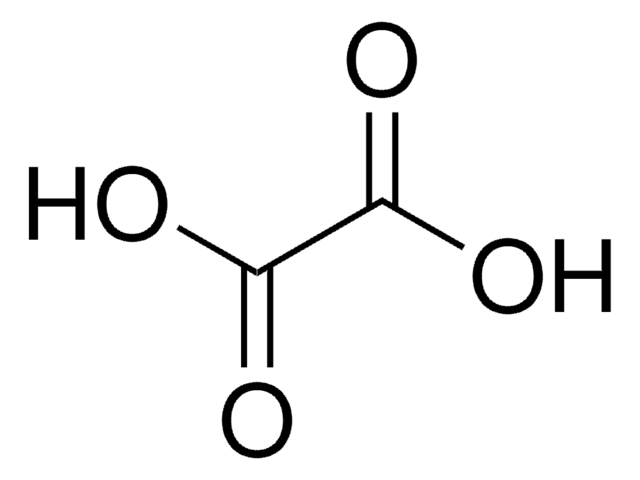
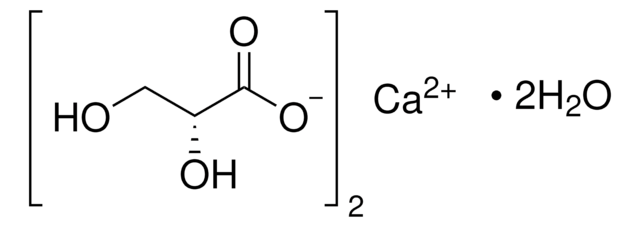
Tartronic acid
≥97.0%
Sinonimo/i:
Hydroxymalonic acid, Hydroxypropanedioic acid
About This Item
Prodotti consigliati
Saggio
≥97.0%
Forma fisica
powder
Punto di fusione
158-160 °C (dec.) (lit.)
Gruppo funzionale
carboxylic acid
hydroxyl
Temperatura di conservazione
2-8°C
Stringa SMILE
OC(C(O)=O)C(O)=O
InChI
1S/C3H4O5/c4-1(2(5)6)3(7)8/h1,4H,(H,5,6)(H,7,8)
ROBFUDYVXSDBQM-UHFFFAOYSA-N
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Applicazioni
- Polymer synthesis for enhanced thermal conductivity: Tartronic acid is used to exploit enzyme reactions in polymer synthesis, significantly increasing the thermal conductivity of materials, which is pivotal in manufacturing and material science applications (Nan et al., 2023).
- Advances in green chemical treatments: This acid plays a role in the electro-oxidation pathways for treating glycerol waste, contributing to sustainable chemical processes and green chemistry applications, which are essential for reducing environmental impact (Cheng et al., 2021).
- Development in biodiesel by-products treatment: Tartronic acid is also involved in kinetic studies for the electrochemical conversion of glycerol, a by-product of biodiesel production, highlighting its role in renewable energy and waste valorization (Pérès et al., 2020).
- Base-free oxidation reactions: It aids in the development of base-free conditions for glycerol to glyceraldehyde oxidation reactions over platinum-based catalysts, offering advancements in catalysis and organic synthesis processes (Capron et al., 2019).
Avvertenze
Warning
Indicazioni di pericolo
Consigli di prudenza
Classi di pericolo
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Organi bersaglio
Respiratory system
Codice della classe di stoccaggio
11 - Combustible Solids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Dispositivi di protezione individuale
dust mask type N95 (US), Eyeshields, Gloves
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.