34660
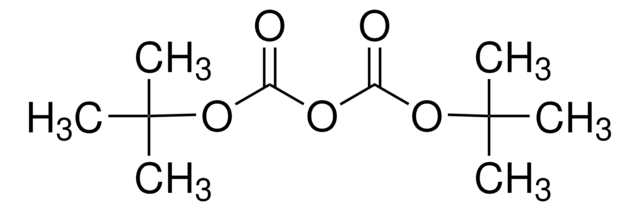
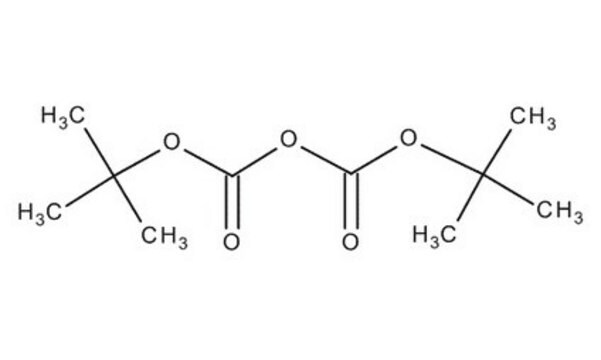
Di-tert-butyl dicarbonate
≥98.0% (GC), for peptide synthesis
Sinonimo/i:
Boc2O, Boc anhydride, Di-tert-butyl pyrocarbonate
About This Item
Prodotti consigliati
product name
Di-tert-butyl dicarbonate, ≥98.0% (GC)
Livello qualitativo
Saggio
≥98.0% (GC)
Forma fisica
solid or liquid
Indice di rifrazione
n20/D 1.409 (lit.)
P. eboll.
56-57 °C/0.5 mmHg (lit.)
Punto di fusione
23 °C (lit.)
Densità
0.95 g/mL at 25 °C (lit.)
applicazioni
peptide synthesis
Temperatura di conservazione
2-8°C
Stringa SMILE
CC(C)(C)OC(=O)OC(=O)OC(C)(C)C
InChI
1S/C10H18O5/c1-9(2,3)14-7(11)13-8(12)15-10(4,5)6/h1-6H3
DYHSDKLCOJIUFX-UHFFFAOYSA-N
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Descrizione generale
Automated Boc protection and deprotection can be done using Synple Automated Synthesis Platform (SYNPLE-SC002), Boc protection cartridges ((SYNPLE-B001), (SYNPLE-B002), and Boc deprotection cartridges (SYNPLE-B011)
Applicazioni
- An azobenzene amino acid(aa).
- N-tert-butoxycarbonyl-3,4-methylenedioxymethamphetamine (t-BOC-MDMA) from MDMA.
It can also be used as a reagent:
- To introduce acid-labile Boc-protecting group in amino acids, peptides, and proteins.
- To prepare styrene derivatives by Heck olefination of aromatic carboxylic acids in the presence of a Pd catalyst.
- To synthesize oxazolidin-2-ones and imidazolidin-2-ones by isocyanation of 1,2-aminoalcohols and 1,2-diamines using DMAP as a catalyst.
- In the preparation of N-tert-butoxycarbonyl-3,4-methylenedioxymethamphetamine (t-Boc-MDMA) from MDMA.
- For the conversion of amines to corresponding isocyanates, carbamates, and urea derivatives.
- In the N-BOC-ylation of amides.
- In the N-BOC-ylation of sensitive compounds under non-aqueous conditions.
Attenzione
Avvertenze
Danger
Indicazioni di pericolo
Classi di pericolo
Acute Tox. 1 Inhalation - Eye Dam. 1 - Flam. Liq. 3 - Skin Irrit. 2 - Skin Sens. 1 - STOT SE 3
Organi bersaglio
Respiratory system
Codice della classe di stoccaggio
3 - Flammable liquids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
98.6 °F - closed cup
Punto d’infiammabilità (°C)
37 °C - closed cup
Dispositivi di protezione individuale
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
Choose from one of the most recent versions:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.