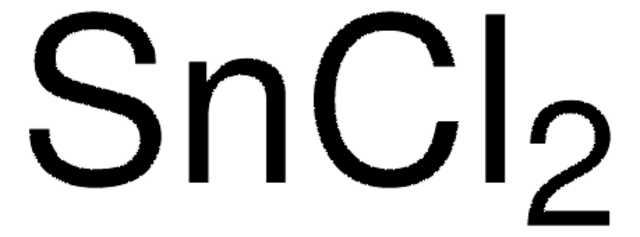204722
Tin(II) chloride
≥99.99% trace metals basis
Sinonimo/i:
Stannous chloride
About This Item
Prodotti consigliati
Grado
for analytical purposes
Livello qualitativo
Tensione di vapore
33 hPa (~429 °C)
Saggio
≥99.99% trace metals basis
Stato
crystalline powder
flakes
Impiego in reazioni chimiche
reagent type: catalyst
core: tin
pH
2.18 (20 °C)
P. ebollizione
652 °C (lit.)
Punto di fusione
246 °C (lit.)
Stringa SMILE
Cl[SnH2]Cl
InChI
1S/2ClH.Sn/h2*1H;/q;;+2/p-2
AXZWODMDQAVCJE-UHFFFAOYSA-L
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Descrizione generale
- aromatic nitro compounds
- nitriles
- cyanosilyl ethers
- organic azides
Applicazioni
- To catalyze the addition of diazo sulfones, diazo phosphine oxides and diazo phosphonates to aldehydes to form β-keto sulfones, β-keto phosphine oxides and β-keto phosphonates, respectively.
- Along with trityl chloride, to catalyze the aldol reaction of silyl enol ethers with acetals or aldehydes and the Michael reaction of silyl enol ethers with α,β-unsaturated ketones.
- As a promoter in the allylic amination of allylic alcohols with amines in the presence of palladium catalyst.
Avvertenze
Danger
Indicazioni di pericolo
Classi di pericolo
Acute Tox. 4 Inhalation - Acute Tox. 4 Oral - Aquatic Chronic 3 - Eye Dam. 1 - Met. Corr. 1 - Skin Corr. 1B - Skin Sens. 1 - STOT RE 2 Oral - STOT SE 3
Organi bersaglio
Cardio-vascular system, Respiratory system
Codice della classe di stoccaggio
8B - Non-combustible corrosive hazardous materials
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Dispositivi di protezione individuale
Eyeshields, Faceshields, Gloves, type P3 (EN 143) respirator cartridges
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.