ROAPRO
Roche
Aprotinin
from bovine lung
Sinonimo/i:
Aprotinin, pancreatic trypsin inhibitor, trypsin inhibitor, pancreas type (bpti), trypsin-kallikrein inhibitor
Autenticatiper visualizzare i prezzi riservati alla tua organizzazione & contrattuali
About This Item
Codice UNSPSC:
12352204
Prodotti consigliati
Origine biologica
bovine lung
Livello qualitativo
Stato
lyophilized
Confezionamento
pkg of 10 mg (10236624001)
pkg of 100 mg (11583794001)
pkg of 50 mg (10981532001)
Produttore/marchio commerciale
Roche
tecniche
electrophoresis: suitable
tissue culture: suitable
Intervallo di pH
3-10
Solubilità
water: soluble 10 mg/mL
Assorbimento
0.84 at 280 nm
Condizioni di spedizione
wet ice
Temperatura di conservazione
2-8°C
Descrizione generale
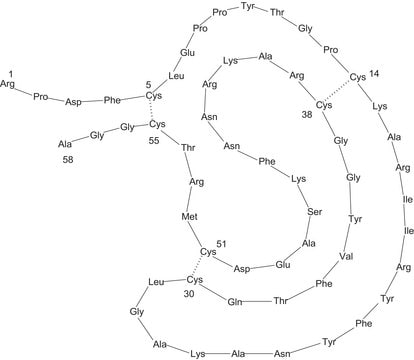
Trypsin inhibitor, pancreas type from bovine lung. It is known as Pancreatic trypsin inhibitor (BPTI). Aprotinin, also known as pancreatic trypsin inhibitor and trypsin-kallikrein inhibitor, is found to be expressed in lungs, spleen, liver, and pancreas. It is also found to be present in the free form in calf serum.
Specificità
Aprotinin inhibits serine proteases. It inhibits kallikrein, the protease that releases hypotensive peptides such as kallidin and bradykinin (human plasma kallikrein: Ki = 3 ×10-8 M at pH 8.0, porcine pancreas kallikrein: Ki = 1 × 10-9 M at pH 8.0), trypsin (Ki = 2.8 × 10-11 M at pH 7.8, Ki = 2.6 × 10-9 M at pH 4.0, non-competetive), trypsinogen, chymotrypsin (Ki = 9 × 10-9 M at pH 8.0), bacterial fibrinolysin, and plasmin (Ki = 1 nM at pH 7.3).
Cathepsin G, acrosin, human leukocyte elastase, and human urokinase are weakly inhibited. Factor Xa, thrombin, subtilisin, papain, pepsin, angiotensin-converting enzyme (ACE), carboxypeptidase A and B, other metalloproteases, and thiolproteases are not inhibited.
Cathepsin G, acrosin, human leukocyte elastase, and human urokinase are weakly inhibited. Factor Xa, thrombin, subtilisin, papain, pepsin, angiotensin-converting enzyme (ACE), carboxypeptidase A and B, other metalloproteases, and thiolproteases are not inhibited.
Applicazioni
Aprotinin is used for the protection of proteins and enzymes during isolation/purification. The inhibition of protease activity increases the lifetime of cells in cell and tissue culture studies.
- Further applications: Purification of urokinase, trypsin, and chymotrypsin on immobilized aprotinin
- Quantification of kallikrein activity in mixtures of esterases and proteases
- Controlled degradation of substrates by avoiding nonspecific proteolysis in clinical chemical tests
- Aprotinin as a model protein in protein-folding studies
- Molecular weight marker in SDS-polyacrylamide gel electrophoresis
Sequenza
Monomeric peptide of 58 amino acids held in conformation by three disulfide bonds.
Definizione di unità
One inhibitor unit (IU) is defined as the amount of aprotinin that completely inhibits 1 U trypsin in < 10 minutes at pH 6. (Trypsin activity determined at +25 °C, pH 8.0, BAEE as substrate).
One inhibitor unit (IU) (+25 °C, BAEE as substrate) corresponds to about 2.8 inhibitor units (+25 °C, Chromozym TRY as substrate).
One inhibitor unit (IU) (+25 °C, BAEE as substrate) corresponds to about 26 kallikrein inhibitor units (KIU) (+25 °C).
One inhibitor unit (IU) (+25 °C, BAEE as substrate) corresponds to about 0.067 inhibitor units (+25 °C; Bz-D,L-Arg-4-Na as substrate, trypsin determination at pH 7.8).
One kallikrein inhibitor unit = 0.17 μg crystalline aprotinin.
One inhibitor unit (IU) (+25 °C, BAEE as substrate) corresponds to about 2.8 inhibitor units (+25 °C, Chromozym TRY as substrate).
One inhibitor unit (IU) (+25 °C, BAEE as substrate) corresponds to about 26 kallikrein inhibitor units (KIU) (+25 °C).
One inhibitor unit (IU) (+25 °C, BAEE as substrate) corresponds to about 0.067 inhibitor units (+25 °C; Bz-D,L-Arg-4-Na as substrate, trypsin determination at pH 7.8).
One kallikrein inhibitor unit = 0.17 μg crystalline aprotinin.
Nota sulla preparazione
Working concentration: 0.06 to 2 μg/ml (0.01 - 0.3 μM)
Working solution: Soluble in water (10 mg/ml) or aqueous buffer solution (e.g., 0.1 M Tris, pH 8.0).
Note: To avoid adsorption of aprotinin onto negatively charged solid phases, e.g., chromatography gels, ultrafiltration membranes, the NaCl concentration should be above 0.1 M or other suitable salts should be added to all buffers used during the separation.
Storage conditions (working solution): -15 to -25 °C
Working solution: Soluble in water (10 mg/ml) or aqueous buffer solution (e.g., 0.1 M Tris, pH 8.0).
Note: To avoid adsorption of aprotinin onto negatively charged solid phases, e.g., chromatography gels, ultrafiltration membranes, the NaCl concentration should be above 0.1 M or other suitable salts should be added to all buffers used during the separation.
Storage conditions (working solution): -15 to -25 °C
Ricostituzione
Freely soluble in water (10 mg/ml) or aqueous buffer solution (e.g., Tris, 0.1 M, pH 8.0). A solution adjusted to pH 7 to 8 is stable for approximately 1 week at 2 to 8 °C.
Aliquots stored at -15 to -25 °C are stable for approximately 6 months.
Note: Avoid repeated freezing and thawing and exposure to strongly alkaline solutions (inactive at pH > 12.8).
Aliquots stored at -15 to -25 °C are stable for approximately 6 months.
Note: Avoid repeated freezing and thawing and exposure to strongly alkaline solutions (inactive at pH > 12.8).
Altre note
For life science research only. Not for use in diagnostic procedures.
Codice della classe di stoccaggio
11 - Combustible Solids
Classe di pericolosità dell'acqua (WGK)
WGK 1
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
[EXPERIMENTS ON THE ISOLATION OF THE KALLIKREIN INACTIVATOR. V. THE ISOLATION OF A KALLIKREIN INACTIVATOR FROM THE BOVINE LUNG AND ITS IDENTIFICATION WITH THE INHIBITOR FROM THE BOVINE PAROTID GLAND].
H KRAUT et al.
Hoppe-Seyler's Zeitschrift fur physiologische Chemie, 338, 231-237 (1964-01-01)
Kamlesh Shroff et al.
Langmuir : the ACS journal of surfaces and colloids, 28(3), 1858-1865 (2011-12-14)
In recent years, a variety of biomimetic constructs have emerged which mimic the bioactive sequences found in the natural extracellular matrix (ECM) proteins such as fibronectin (FN) that promote cell adhesion as well as proliferation on artificially functionalized interfaces. Much
Glucose Starvation Increases V-ATPase Assembly and Activity in Mammalian Cells through AMP
Kinase and Phosphatidylinositide 3-Kinase/Akt Signaling
Kinase and Phosphatidylinositide 3-Kinase/Akt Signaling
Christina M. McGuire and Michael Forgac
The Journal of Biological Chemistry (2018)
Selecting protein N-terminal peptides by combined
fractional diagonal chromatography
fractional diagonal chromatography
An S, et al.
Nature Protocols (2011)
H Fritz et al.
Hoppe-Seyler's Zeitschrift fur physiologische Chemie, 360(3), 437-444 (1979-03-01)
Using the indirect immunofluorescence technique, the basic kallikrein-trypsin inhibitor of bovine organs, Trasylol, could be localized in tissue mast cells of bovine lung, liver, pancreas and parotid gland. Identification of cells exhibiting specific fluorescence as tissue mast cells was achieved
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.