MABN687
Anti-β-amyloid fibril-specific, clone B10, AP Antibody
clone B10, from camel, alkaline phosphatase conjugate
Sinonimo/i:
Amyloid beta A4 protein, ABPP, APPI, APP, Alzheimer disease amyloid protein, Cerebral vascular amyloid peptide, CVAP, PreA4, Protease nexin-II, PN-II, N-APP2.Soluble APP-alpha, S-APP-alpha, Soluble APP-beta, S-APP-beta, C99, Beta-amyloid protein 42, Beta
About This Item
Prodotti consigliati
Origine biologica
camel
Livello qualitativo
Coniugato
alkaline phosphatase conjugate
Forma dell’anticorpo
purified immunoglobulin
Tipo di anticorpo
primary antibodies
Clone
B10, monoclonal
Reattività contro le specie
human
tecniche
ELISA: suitable
dot blot: suitable
immunofluorescence: suitable
immunohistochemistry: suitable
immunoprecipitation (IP): suitable
Isotipo
IgG
N° accesso NCBI
N° accesso UniProt
Condizioni di spedizione
dry ice
modifica post-traduzionali bersaglio
unmodified
Informazioni sul gene
human ... APP(351)
Descrizione generale
Immunogeno
Applicazioni
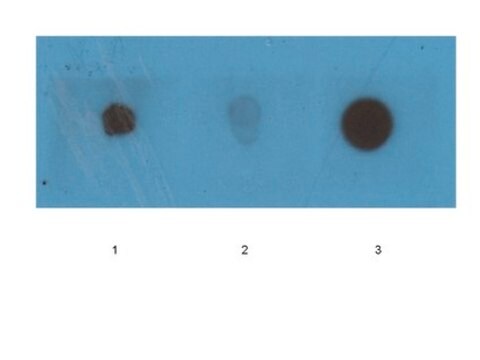
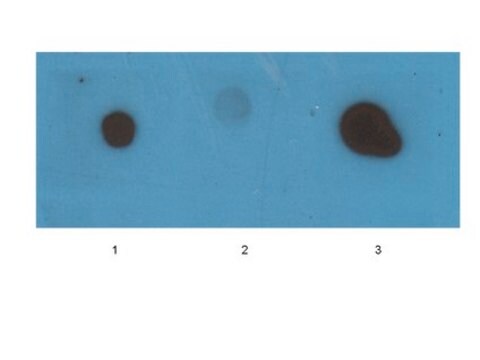
Dot Blot Analysis: A representative lot detected β-amyloid fibril-specific in synthetic Aβ (1–40) peptide (Habicht, G., et al. (2007). PNAS. 104(49):19232-19237).
Dot Blot Analysis: A representative lot detected β-amyloid fibril-specific in chemically modified fibrils (Haupt, C., et al. (2011). J. Mole. Biol. 405:341-348).
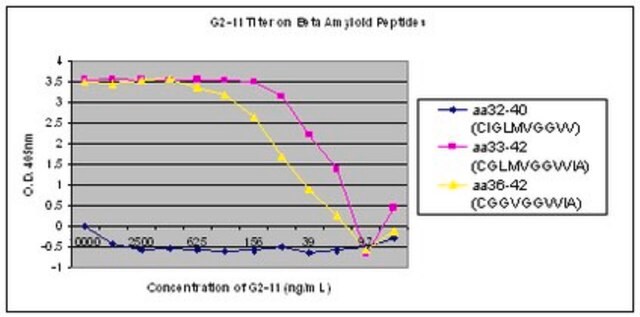
Elisa Analysis: A representative lot detected β-amyloid fibril-specific in N-biotinylated Aβ (1–40) conformers (disaggregated peptide, oligomers, or fibrils) (Morgado, I., et al. (2012). PNAS. 109(31):12503-12508).
Immunohistochemistry Analysis: A representative lot detected β-amyloid fibril-specific in Hippocampal sections from Alzheimer brain tissue (Habicht, G., et al. (2007). PNAS. 104(49):19232-19237).
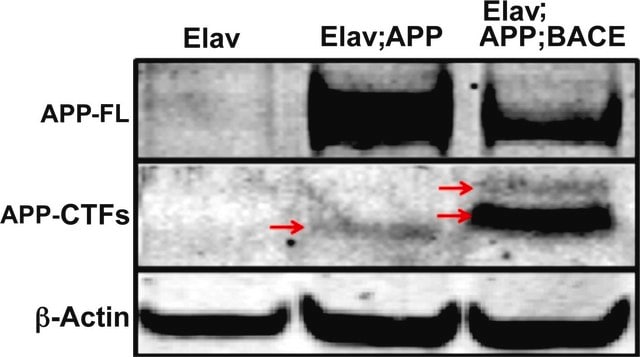
Immunoprecipitation Analysis: A representative lot detected β-amyloid fibril-specific in native soluble and dispersible fractions from the brain lysates (Upadhaya, A.R., et al. (2014). BRAIN. 1-17).
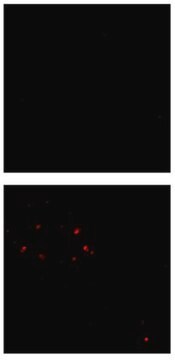
Immunofluorescence Analysis: A representative lot detected β-amyloid fibril-specific in cell culture-derived amyloid plaques (Habicht, G., et al. (2007). PNAS. 104(49):19232-19237).
Qualità
Immunohistochemistry Analysis: A 1:50 dilution of this antibody detected β-amyloid fibril-specific in human Alzheimer′s brain tissue.
Stato fisico
Note: This is a Camelid antibody fused to an alkaline phosphatase and does not require a secondary antibody for detection.
Altre note
Non trovi il prodotto giusto?
Prova il nostro Motore di ricerca dei prodotti.
Codice della classe di stoccaggio
12 - Non Combustible Liquids
Classe di pericolosità dell'acqua (WGK)
nwg
Certificati d'analisi (COA)
Cerca il Certificati d'analisi (COA) digitando il numero di lotto/batch corrispondente. I numeri di lotto o di batch sono stampati sull'etichetta dei prodotti dopo la parola ‘Lotto’ o ‘Batch’.
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.