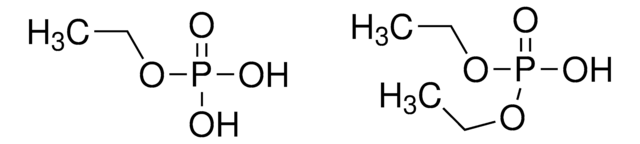8.07471
Polyphosphoric acid
for synthesis
Sinonimo/i:
Polyphosphoric acid, PPA
About This Item
Prodotti consigliati
Grado
for synthesis
Livello qualitativo
Tensione di vapore
2 hPa ( 20 °C)
Saggio
83-87% P2O5 basis (acidimetric)
Stato
viscous liquid
P. ebollizione
530 °C/1013 hPa
Punto di fusione
-20 °C
Densità
2.06 g/cm3 at 20 °C
Temperatura di conservazione
2-30°C
Stringa SMILE
[P](=O)(O)(O)O.[p]21[o][p]([o][o]2)[o][o]1
InChI
1S/O5P2.H3O4P/c1-2-7-4-3-6(1)5-7;1-5(2,3)4/h;(H3,1,2,3,4)
FVYIVXLIMHNYTD-UHFFFAOYSA-N
Descrizione generale
Applicazioni
- In acylation and alkylation reactions.
- To synthesize cyclic aromatic compounds via cyclization of aromatic acids, esters, ketones, aldehydes, acetals, alcohols, and alkenes.
- In Bischler−Napieralski reaction to construct isoquinoline ring system from phenethylformamides.
- To prepare substituted indoles at position-2 from hydrazones via Fisher indole synthesis.
- For the conversion of oximes into amides via Beckmann rearrangement.
PPA can also be used as a catalyst to synthesize:
- Aniline derivatives by Lossen rearrangement of aromatic carboxylic acids with nitromethane.
- Amides by hydrolysis nitriles.
- Dimethyl carbonate (DMC) from urea and methanol.
- Benzofurans by cyclization of α-phenoxy ketones.
PPA is also used with other reagents for various organic transformations. For example:
- PPA/Ethanethiol mixture can be used to selectively open one epoxide in bis-epoxy steroid.
- PPA/POCl3 catalytic mixture used to convert tertiary alcohols into corresponding chlorides.
- PPA/acetic acid is used to synthesize nonenolizable β-diketones.
- PPA/potassium iodide mixture used to convert primary methyl ether to a primary alkyl iodide.
- PPA/nitric acid catalytic combination used for nitration reactions which is less hazardous than Ac2O/HNO3 mixtures.
Risultati analitici
Due to its specific melting range the product may be solid, liquid, a solidified melt or a supercooled melt.
Avvertenze
Danger
Indicazioni di pericolo
Classi di pericolo
Eye Dam. 1 - Met. Corr. 1 - Skin Corr. 1B
Codice della classe di stoccaggio
8B - Non-combustible, corrosive hazardous materials
Classe di pericolosità dell'acqua (WGK)
WGK 1
Punto d’infiammabilità (°F)
does not flash
Punto d’infiammabilità (°C)
does not flash
Certificati d'analisi (COA)
Cerca il Certificati d'analisi (COA) digitando il numero di lotto/batch corrispondente. I numeri di lotto o di batch sono stampati sull'etichetta dei prodotti dopo la parola ‘Lotto’ o ‘Batch’.
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.