219362
Cathepsin B, Human Liver
Cathepsin B, Human Liver, CAS 9047-22-7, is a purified native cathepsin B from human liver, purified by affinity chromatography. Upregulated in many types of tumors.
Sinonimo/i:
Cathepsin B, Human Liver, cat B, cysteine cathepsin
About This Item
Prodotti consigliati
Origine biologica
human liver
Livello qualitativo
Saggio
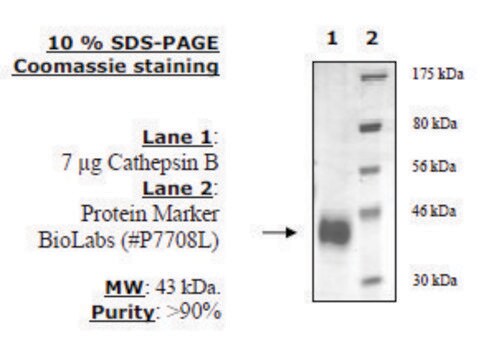
≥95% (SDS-PAGE)
Stato
liquid
Attività specifica
≥10 units/mg protein
Purificato mediante
affinity chromatography
Produttore/marchio commerciale
Calbiochem®
Condizioni di stoccaggio
OK to freeze
avoid repeated freeze/thaw cycles
tecniche
activity assay: suitable
Compatibilità
suitable for molecular biology
applicazioni
life science and biopharma
Condizioni di spedizione
wet ice
Temperatura di conservazione
−70°C
Informazioni sul gene
human ... CTSB(1508)
Descrizione generale
Native cathepsin B from human liver, purified by affinity chromatography and HPLC. The most investigated enzyme of all lysosomal cysteine proteases. Cathepsin B belongs to the papain-like family of cysteine proteases and is produced as a preproenzyme. It is a bilobal protein, and its catalytic site is situated at the interface between the two lobes.
Applicazioni
- Diagnostics: as a potent and independent prognostic marker for endometrial cancer, pancreatic adenocarcinoma, and inflammatory disease.
- Drug development: during the neovascularization process and as a potent therapeutic target for various pathologies, cancer progression, and osteoarthritis in humans.
- Pharmacology: for increasing the therapeutic index of doxorubicin by incorporating the cathepsin B cleavable spacer Phe-Lys-4-aminobenzyloxycarbonyl into an albumin-binding prodrug.
- Molecular biology: in cathepsin B activity assay.
Azioni biochim/fisiol
Attenzione
Definizione di unità
Stato fisico
Nota sulla preparazione
Ricostituzione
Altre note
Kostoulas, G., et al. 1999. FEBS Lett.455, 286.
Strojnik, T., et al. 1999. Clin. Cancer Res.5, 559.
Maquire, T.M., et al. 1998. Int. J. Biol. Markers13, 139.
Berquim, I.M., and Sloane, B.F. 1996. Adv. Exp. Med. Biol.389, 281.
Note legali
Codice della classe di stoccaggio
11 - Combustible Solids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Certificati d'analisi (COA)
Cerca il Certificati d'analisi (COA) digitando il numero di lotto/batch corrispondente. I numeri di lotto o di batch sono stampati sull'etichetta dei prodotti dopo la parola ‘Lotto’ o ‘Batch’.
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.