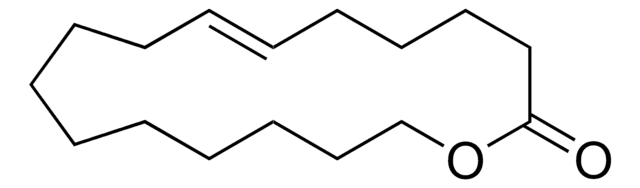
W241504
Ethyl acetoacetate
≥99%, FCC, FG
Sinonimo/i:
Acetoacetic ester
About This Item
Prodotti consigliati
Origine biologica
synthetic
Livello qualitativo
Grado
FG
Kosher
agenzia
meets purity specifications of JECFA
Conformità normativa
EU Regulation 1334/2008 & 178/2002
FCC
FDA 21 CFR 117
FDA 21 CFR 172.515
Densità del vapore
4.48 (vs air)
Tensione di vapore
1 mmHg ( 28.5 °C)
Saggio
≥99%
Temp. autoaccensione
580 °F
Limite di esplosione
9.5 %
Indice di rifrazione
n20/D 1.418-1.421
P. ebollizione
181 °C (lit.)
Punto di fusione
−43 °C (lit.)
Solubilità
water: soluble 130 g/L at 20 °C
Densità
1.029 g/mL at 20 °C (lit.)
applicazioni
flavors and fragrances
Documentazione
see Safety & Documentation for available documents
Allergene alimentare
no known allergens
Organolettico
apple; fatty; green; fruity
Stringa SMILE
CCOC(=O)CC(C)=O
InChI
1S/C6H10O3/c1-3-9-6(8)4-5(2)7/h3-4H2,1-2H3
XYIBRDXRRQCHLP-UHFFFAOYSA-N
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Categorie correlate
Applicazioni
- Fabrication of a novel magnetic nanostructure based on cellulose-gellan gum hydrogel, embedded with MgAl LDH as an efficient catalyst for the synthesis of polyhydroquinoline derivatives.: This study explores the use of ethyl acetoacetate in the synthesis of polyhydroquinoline derivatives, showcasing its application in developing efficient catalytic systems for organic reactions (Hjazi A, 2024).
- Evaluation of diethyl 4-(5-bromo-1H-indol-3-yl)-2,6-dimethyl-1,4-dihydropyridine-3,5-dicarboxylate: synthesis, anti-corrosion potential, and biomedical applications.: This research investigates the biomedical applications and anti-corrosion properties of compounds synthesized using ethyl acetoacetate, emphasizing its versatility in chemical synthesis and material science (Ahamed FMM et al., 2024).
- Fe(3)O(4) nanoparticles impregnated eggshell as an efficient biocatalyst for eco-friendly synthesis of 2-amino thiophene derivatives.: The study highlights the use of ethyl acetoacetate in green chemistry, particularly in the eco-friendly synthesis of thiophene derivatives using biocatalysts (Zargari M et al., 2024).
- Pyrano[2,3-c]pyrazole fused spirooxindole-linked 1,2,3-triazoles as antioxidant agents: Exploring their utility in the development of antidiabetic drugs via inhibition of α-amylase and DPP4 activity.: This paper discusses the synthesis of novel compounds with antidiabetic properties using ethyl acetoacetate, demonstrating its potential in drug development (Chahal S et al., 2024).
- Access to Functionalized Cyclohex-2-enones from a Multicomponent Cascade Reaction of Readily Available Alkynes, Ketones, and Ethyl Acetoacetate.: The research details a multicomponent cascade reaction involving ethyl acetoacetate, highlighting its utility in the efficient synthesis of functionalized cyclohexenones (Jiang D et al., 2024).
Codice della classe di stoccaggio
10 - Combustible liquids
Classe di pericolosità dell'acqua (WGK)
WGK 1
Punto d’infiammabilità (°F)
164.3 °F - closed cup
Punto d’infiammabilità (°C)
73.5 °C - closed cup
Dispositivi di protezione individuale
Eyeshields, Gloves, type ABEK (EN14387) respirator filter
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.