M51407
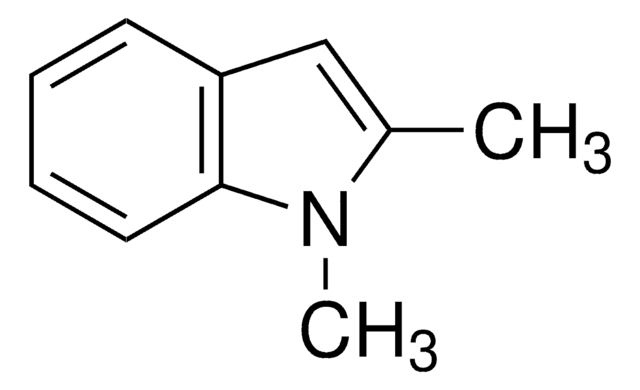
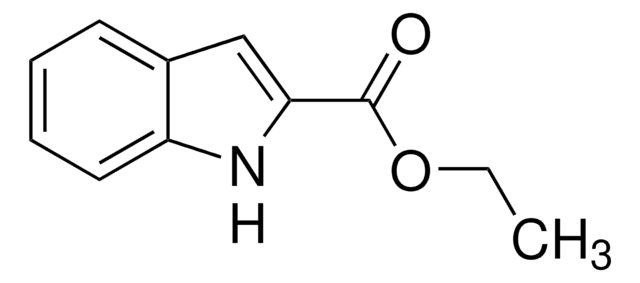
2-Methylindole
98%
Sinonimo/i:
NSC 7514
Autenticatiper visualizzare i prezzi riservati alla tua organizzazione & contrattuali
About This Item
Formula empirica (notazione di Hill):
C9H9N
Numero CAS:
Peso molecolare:
131.17
Beilstein:
109781
Numero CE:
Numero MDL:
Codice UNSPSC:
12352100
ID PubChem:
NACRES:
NA.22
Prodotti consigliati
Saggio
98%
P. ebollizione
273 °C (lit.)
Punto di fusione
57-59 °C (lit.)
Stringa SMILE
Cc1cc2ccccc2[nH]1
InChI
1S/C9H9N/c1-7-6-8-4-2-3-5-9(8)10-7/h2-6,10H,1H3
BHNHHSOHWZKFOX-UHFFFAOYSA-N
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Applicazioni
Reactant for:
- Regioselective synthesis of oxopyrrolidine analogs via iodine-catalyzed Markovnikov addition reaction
- Friedel-Crafts alkylation reactions
- Preparation of tryptophan dioxygenase inhibitors pyridyl-ethenyl-indoles as potential anticancer immunomodulators
- Preparation of plant-growth inhibitors
- Michael addition reactions
- Synthesis of cyclooxygenase-1 (COX-1)/cyclooxygenase-2 (COX-2) inhibitors
Avvertenze
Warning
Indicazioni di pericolo
Consigli di prudenza
Classi di pericolo
Acute Tox. 4 Oral
Codice della classe di stoccaggio
11 - Combustible Solids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
285.8 °F
Punto d’infiammabilità (°C)
141 °C
Dispositivi di protezione individuale
dust mask type N95 (US), Eyeshields, Gloves
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
T Bhattacharya et al.
Spectrochimica acta. Part A, Molecular and biomolecular spectroscopy, 60(8-9), 1957-1966 (2004-07-14)
Electrochemical measurements by cyclic voltammetry predict the possibility of occurrence of photoinduced electron-transfer (PET) reactions between the ground state of 2-phenylindole (2PI) (electron donor) and the excited singlet of 9-cyanoanthracene (9CNA) molecule acting as an electron acceptor. However, 2PI should
N G Faleev et al.
Biochemistry and molecular biology international, 35(5), 1037-1040 (1995-04-01)
Tryptophanase was generally considered to be inactive towards tryptophan derivatives substituted at 2-position of the indole ring. We have shown that cells containing tryptophanase catalyze the formation of 2-methyl-L-tryptophan from 2-methylindole and L-serine, and from 2-methylindole, pyruvate and ammonium ion.
Emma L Harry et al.
The Analyst, 136(8), 1728-1732 (2011-02-26)
The potential of ion mobility (IM) spectrometry in combination with mass spectrometry (MS) for real-time reaction monitoring is reported. The combined IM-MS approach using electrospray ionization affords gas-phase analyte characterization based on both mass-to-charge (m/z) ratio and gas-phase ion mobility
T Misra et al.
Spectrochimica acta. Part A, Molecular and biomolecular spectroscopy, 58(8), 1631-1641 (2002-08-09)
By using steady state and time-resolved (laser flash photolysis and single photon counting) spectroscopic techniques the quenching of the lowest excited singlet (S1) state of 9-cyanoanthracene (9CNA) by the donors (quenchers) 2-methylindole (2MI) and 2-methylindoline (2MIN) in solvents of different
[2-Methylindoles substituted in the 1st, 3d and 5th positions and the diffuse neuroendocrine APUD system].
K S Shadurskiĭ et al.
Farmakologiia i toksikologiia, 46(2), 115-120 (1983-03-01)
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.