222410
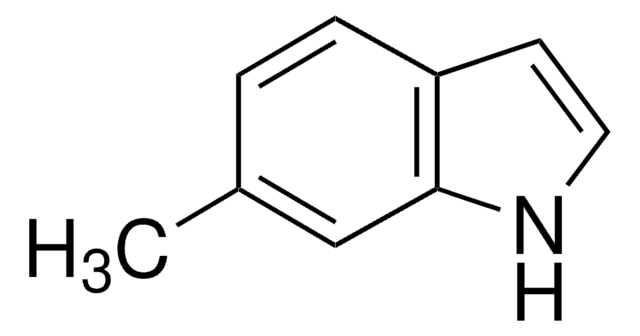
5-Methylindole
99%
Sinonimo/i:
NSC 522562
Autenticatiper visualizzare i prezzi riservati alla tua organizzazione & contrattuali
About This Item
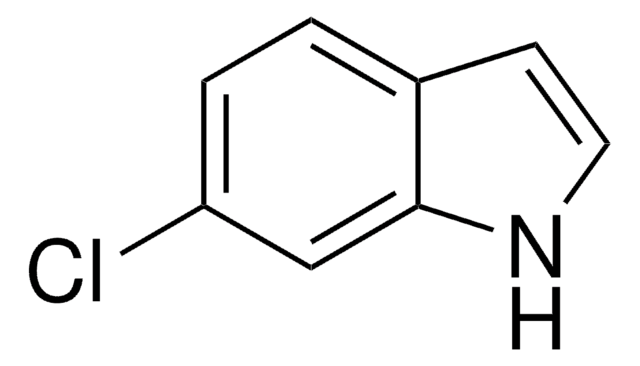
Formula empirica (notazione di Hill):
C9H9N
Numero CAS:
Peso molecolare:
131.17
Numero CE:
Numero MDL:
Codice UNSPSC:
12352100
ID PubChem:
NACRES:
NA.22
Prodotti consigliati
Livello qualitativo
Saggio
99%
Stato
solid
Punto di fusione
60-62 °C (lit.)
Stringa SMILE
Cc1ccc2[nH]ccc2c1
InChI
1S/C9H9N/c1-7-2-3-9-8(6-7)4-5-10-9/h2-6,10H,1H3
YPKBCLZFIYBSHK-UHFFFAOYSA-N
Categorie correlate
Descrizione generale
The binding of 5-methylindole (inducer) to the Escherichia coli trp repressor has been studied. The mass analyzed threshold ionization spectra of jetcooled 5-methylindole (5MI) has also been studied.
Applicazioni
Reactant for preparation of:
- Pharmaceutically active 2-oxo-1-pyrrolidine analogues
- Potential anticancer immunomodulators
- Preparation of antifungal agents
- Sodium-dependent glucose co-transporter 2 (SGLT2) inhibitors for the management of hyperglycemia in diabetes
- IL2-inducible T-cell kinase (ITK) inhibitors
- Checkpoint 1 kinase inhibitors
- CRTh2 antagonists
- Inhibitors of human immunodeficiency virus type 1 (HIV-1) attachment
- Agonists of the histamine H4 receptor
- Monoamine reuptake inhibitors
Avvertenze
Warning
Indicazioni di pericolo
Consigli di prudenza
Classi di pericolo
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Organi bersaglio
Respiratory system
Codice della classe di stoccaggio
11 - Combustible Solids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Dispositivi di protezione individuale
dust mask type N95 (US), Eyeshields, Gloves
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
Changjiang Dong et al.
Science (New York, N.Y.), 309(5744), 2216-2219 (2005-10-01)
Chlorinated natural products include vancomycin and cryptophycin A. Their biosynthesis involves regioselective chlorination by flavin-dependent halogenases. We report the structural characterization of tryptophan 7-halogenase (PrnA), which regioselectively chlorinates tryptophan. Tryptophan and flavin adenine dinucleotide (FAD) are separated by a 10
Jung Lee Lin et al.
The Journal of chemical physics, 120(11), 5057-5063 (2004-07-23)
The vibrationally resolved mass analyzed threshold ionization spectra of jetcooled 5-methylindole (5MI) and 3-methylindole (3MI) have been recorded by ionizing via various vibronic levels of each species. The adiabatic ionization energies (IEs) of 5MI and 3MI are determined to be
P Babitzke et al.
The Journal of biological chemistry, 270(21), 12452-12456 (1995-05-26)
A filter binding assay was used to determine the structural features of L-tryptophan required for activation of TRAP, the trp RNA-binding attenuation protein of Bacillus subtilis. We examined the ability of L-tryptophan and 26 of its analogs to activate TRAP.
F Peter Guengerich et al.
Journal of medicinal chemistry, 47(12), 3236-3241 (2004-05-28)
Indigoids, a class of bis-indoles, represent a promising protein kinase inhibitor scaffold. Oxidation of indole by cytochrome P450 (P450) has been shown to generate species (indoxyl, isatin) that couple to yield indigo and indirubin. Escherichia coli-expressed human P450 2A6 mutants
Dorleta Gonzalez et al.
Bioorganic & medicinal chemistry, 26(9), 2551-2560 (2018-04-17)
Following the premises of the multitarget-directed ligands approach for the drug R&D against neurodegenerative diseases, where Alzheimer's disease (AD) outstands, we have synthesized and evaluated analogues of the gramine derivative ITH12657 (1-benzyl-5-methyl-3-(piperidin-1-ylmethyl-1H-indole, 2), which had shown important neuroprotective properties, such
Global Trade Item Number
| SKU | GTIN |
|---|---|
| 222410-250MG | |
| 222410-1G | 4061838777966 |
| 222410-500G |
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.