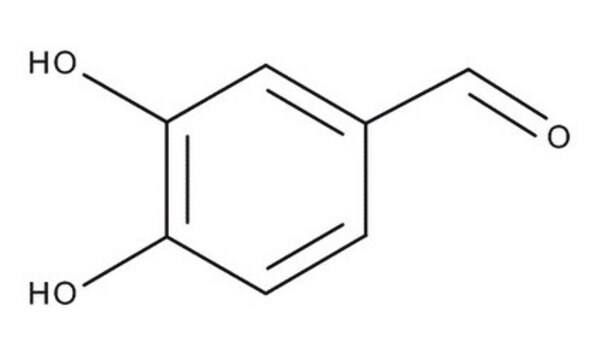
D108405
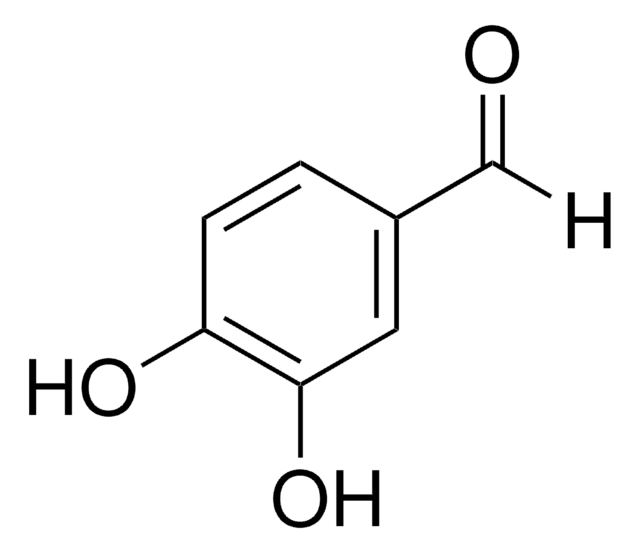
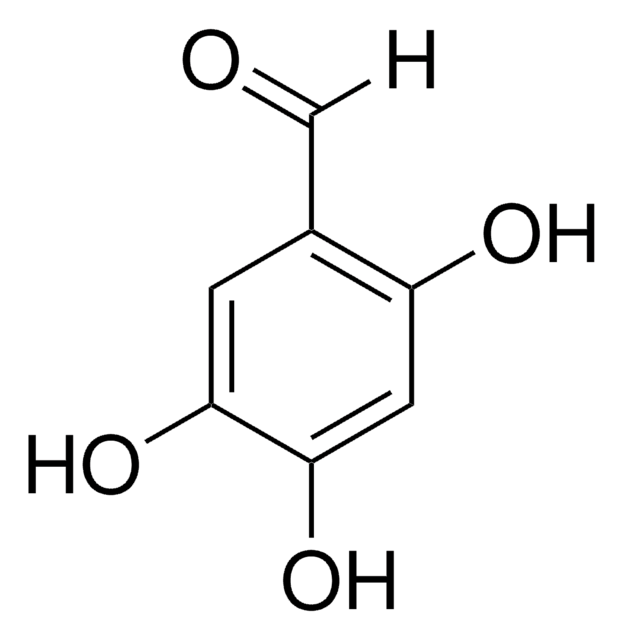
3,4-Dihydroxybenzaldehyde
97%
Sinonimo/i:
Protocatechualdehyde
Autenticatiper visualizzare i prezzi riservati alla tua organizzazione & contrattuali
About This Item
Formula condensata:
(HO)2C6H3CHO
Numero CAS:
Peso molecolare:
138.12
Beilstein:
774381
Numero CE:
Numero MDL:
Codice UNSPSC:
12352100
ID PubChem:
NACRES:
NA.22
Prodotti consigliati
Livello qualitativo
Saggio
97%
Stato
powder
Punto di fusione
150-157 °C (lit.)
Stringa SMILE
Oc1ccc(C=O)cc1O
InChI
1S/C7H6O3/c8-4-5-1-2-6(9)7(10)3-5/h1-4,9-10H
IBGBGRVKPALMCQ-UHFFFAOYSA-N
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Applicazioni
3,4-Dihydroxybenzaldehyde can be used as a reactant for the synthesis of:
- Copolymers containing poly(p-phenylenevinylene) chromophore to be used in light-emitting electrochemical cell.
- 2-Arylbenzothiazoles with potential application as anti-cancer agents against human colon cancer cells.
- Variety of thiazolidin-4-one ring systems having antimicrobial activity.
- Bis-Schiff bases of isatins which can be used as antiglycating agents.
Avvertenze
Warning
Indicazioni di pericolo
Consigli di prudenza
Classi di pericolo
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Organi bersaglio
Respiratory system
Codice della classe di stoccaggio
11 - Combustible Solids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Dispositivi di protezione individuale
dust mask type N95 (US), Eyeshields, Gloves
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
Synthesis of bis-Schiff bases of isatins and their antiglycation activity.
Khan KM, et al.
Bioorganic & Medicinal Chemistry, 17(22), 7795-7801 (2009)
Synthesis of new bioactive venlafaxine analogs: Novel thiazolidin-4-ones as antimicrobials.
Kavitha CV, et al.
Bioorganic & Medicinal Chemistry, 14(7), 2290-2299 (2006)
Synthesis and electroluminescence of novel copolymers containing crown ether spacers.
Sun Q, et al.
Journal of Materials Chemistry, 13(4), 800-806 (2003)
Catriona G Mortimer et al.
Journal of medicinal chemistry, 49(1), 179-185 (2006-01-06)
A series of new 2-phenylbenzothiazoles has been synthesized on the basis of the discovery of the potent and selective in vitro antitumor properties of 2-(3,4-dimethoxyphenyl)-5-fluorobenzothiazole (8n; GW 610, NSC 721648). Synthesis of analogues substituted in the benzothiazole ring was achieved
Narsimha Mamidi et al.
The journal of physical chemistry. B, 116(35), 10684-10692 (2012-08-07)
Diacylglycerol (DAG) regulates a broad range of cellular functions including tumor promotion, apoptosis, differentiation, and growth. Thus, the DAG-responsive C1 domain of protein kinase C (PKC) isoenzymes is considered to be an attractive drug target for the treatment of cancer
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.