260843
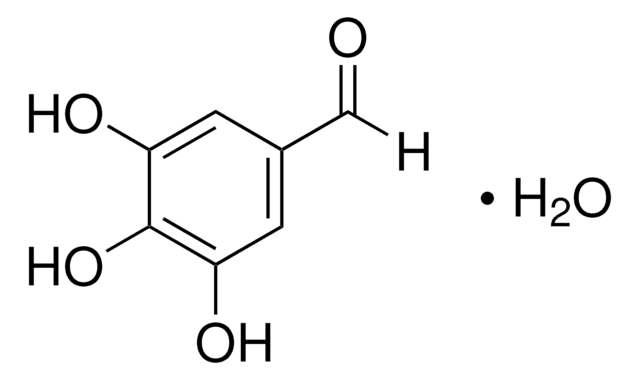
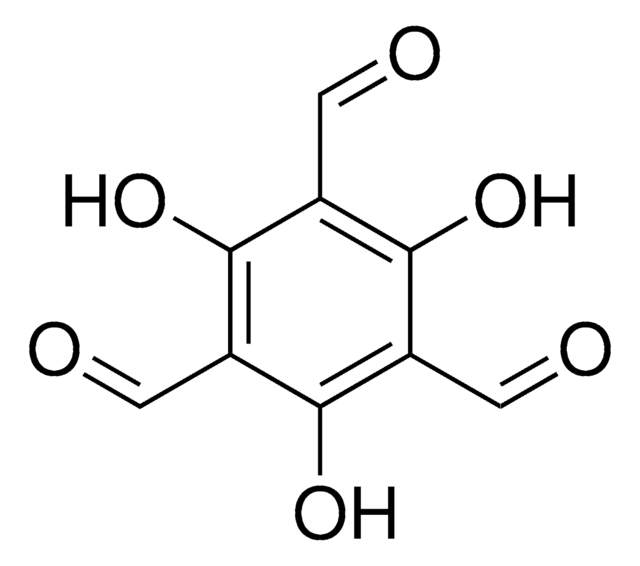
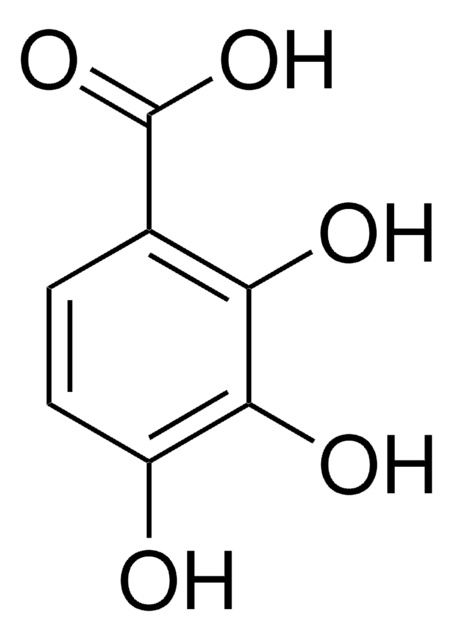
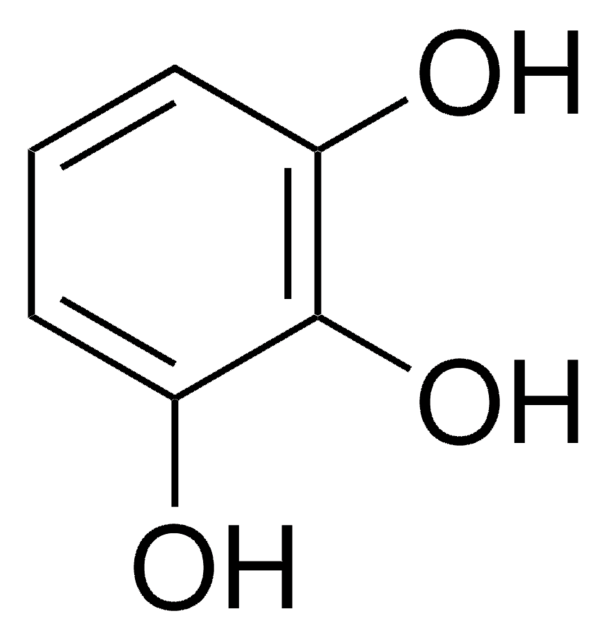
2,3,4-Trihydroxybenzaldehyde
98%
Sinonimo/i:
Pyrogallol-4-carboxaldehyde
Autenticatiper visualizzare i prezzi riservati alla tua organizzazione & contrattuali
About This Item
Formula condensata:
(HO)3C6H2CHO
Numero CAS:
Peso molecolare:
154.12
Beilstein:
2328658
Numero CE:
Numero MDL:
Codice UNSPSC:
12352100
ID PubChem:
NACRES:
NA.22
Prodotti consigliati
Livello qualitativo
Saggio
98%
Stato
powder
Punto di fusione
159-161 °C (lit.)
Stringa SMILE
[H]C(=O)c1ccc(O)c(O)c1O
InChI
1S/C7H6O4/c8-3-4-1-2-5(9)7(11)6(4)10/h1-3,9-11H
CRPNQSVBEWWHIJ-UHFFFAOYSA-N
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Categorie correlate
Descrizione generale
Antimicrobial activity of carbohydrazone derived from 2,3,4-trihydroxybenzaldehyde against bacteria and fungi has been investigated. 2,3,4-trihydroxybenzaldehyde forms Schiff bases via [1+1] condensation with anthranilic acid.
Applicazioni
2,3,4-Trihydroxybenzaldehyde has been used in the preparation of 1,5-dimethyl-2-phenyl-4-[(1E)-(2,3,4-trihydroxybenzylidene)amino]-1H-pyrazol-3(2H)-one.
Avvertenze
Warning
Indicazioni di pericolo
Consigli di prudenza
Classi di pericolo
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Organi bersaglio
Respiratory system
Codice della classe di stoccaggio
11 - Combustible Solids
Classe di pericolosità dell'acqua (WGK)
WGK 2
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Dispositivi di protezione individuale
dust mask type N95 (US), Eyeshields, Gloves
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
Yuan Chen et al.
International journal of biological macromolecules, 143, 714-723 (2019-11-15)
In this study, the structure of inulin was chemically modified by Schiff bases in order to improve its biological activity. A total of 6 kinds of inulin derivatives were synthesized according to aza-Wittig reaction. Their structures were confirmed by FTIR
Eila Pelttari et al.
Zeitschrift fur Naturforschung. C, Journal of biosciences, 62(7-8), 483-486 (2007-10-05)
Certain substituted salicylaldehydes are known to have highly potent antimicrobial activity against bacteria and fungi, but the mechanism underlying this remarkable activity is not known, and almost nothing has been reported on the effects of further modification of the structures
B D Vázquez-Cabral et al.
Chemico-biological interactions, 272, 1-9 (2017-05-10)
Black tea infusion is the common substrate for preparing kombucha; however other sources such as oak leaves infusions can be used for the same purpose. Almost any white oak species have been used for medicinal applications by some ethnic groups
Sari Honda et al.
Free radical biology & medicine, 106, 228-235 (2017-02-23)
In this study, the mechanism of the xanthine oxidase (XO) inhibitory activity of pyrogallol, the main inhibitor found in roasted coffee, was investigated. Pyrogallol was unstable and readily converted to purpurogallin in a pH 7.4 solution, a physiological model of
1, 5-Dimethyl-2-phenyl-4-[(1E)-(2, 3, 4-trihydroxybenzylidene) amino]-1H-pyrazol-3 (2H)-one.
Sun Y-F, et al.
Acta Crystallographica Section E, Structure Reports Online, 63(5), o2522-o2523 (2007)
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.