C70401
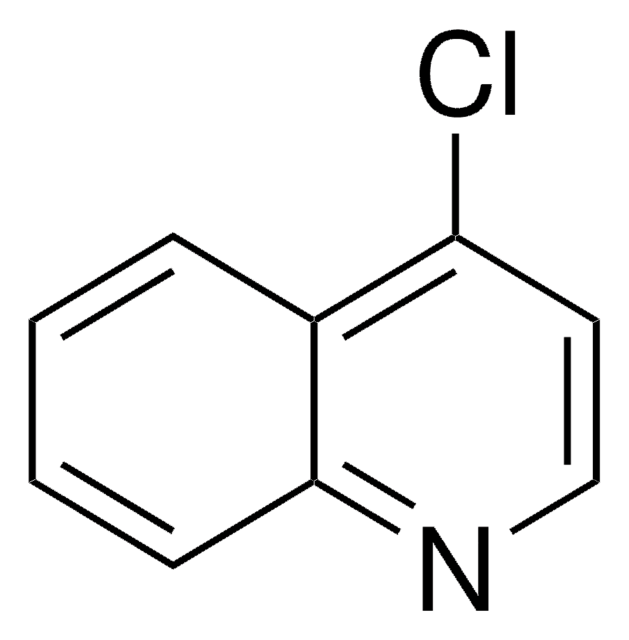
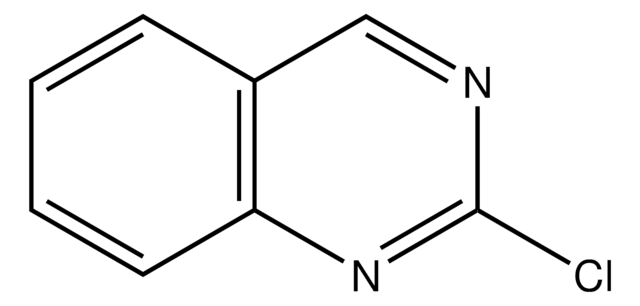
2-Chloroquinoline
99%
Autenticatiper visualizzare i prezzi riservati alla tua organizzazione & contrattuali
About This Item
Formula empirica (notazione di Hill):
C9H6ClN
Numero CAS:
Peso molecolare:
163.60
Beilstein:
112561
Numero CE:
Numero MDL:
Codice UNSPSC:
12352100
ID PubChem:
NACRES:
NA.22
Prodotti consigliati
Saggio
99%
Stato
crystals
P. ebollizione
266-267 °C (lit.)
Punto di fusione
34-37 °C (lit.)
Densità
1.23 g/mL at 25 °C (lit.)
Stringa SMILE
Clc1ccc2ccccc2n1
InChI
1S/C9H6ClN/c10-9-6-5-7-3-1-2-4-8(7)11-9/h1-6H
OFUFXTHGZWIDDB-UHFFFAOYSA-N
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Avvertenze
Warning
Indicazioni di pericolo
Consigli di prudenza
Classi di pericolo
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Organi bersaglio
Respiratory system
Codice della classe di stoccaggio
11 - Combustible Solids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
230.0 °F - closed cup
Punto d’infiammabilità (°C)
110 °C - closed cup
Dispositivi di protezione individuale
dust mask type N95 (US), Eyeshields, Gloves
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
K Shiva Kumar et al.
Bioorganic & medicinal chemistry, 20(7), 2199-2207 (2012-03-06)
A number of 2-(1H-indol-3-yl)quinoline-3-carbonitrile derivatives were synthesized via AlCl(3)-mediated C-C bond forming reaction between 2-chloroquinoline-3-carbonitrile and various indoles. The methodology does not require any N-protection of the indoles employed and provided the corresponding products in good yields. The molecular structure
Santosh Kumar et al.
International journal of biological macromolecules, 49(3), 356-361 (2011-06-07)
This paper describes an elegant cross-linking technique for the preparation of chitosan-chloroquinoline derivative by using a greener technique. Chitosan solution in aqueous acetic acid was treated with 2-chloroquinoline-3-carbaldehyde solution to form hydrogel; the resulting hydrogel was subjected to solvent exchange.
S Fetzner et al.
FEMS microbiology letters, 112(2), 151-157 (1993-09-01)
Resting cells of Pseudomonas putida strain 86 were grown on quinoline transformed 2-chloroquinoline to 2-chloro-cis-7,8-dihydro-7,8-dihydroxyquinoline which was not converted further. 7,8-Dioxygenating activity was present when the enzymes of quinoline catabolism were induced. Quinoline-grown cells of strain 86 treated simultaneously with
Krzysztof Marciniec et al.
Journal of chromatographic science, 55(9), 934-939 (2017-06-28)
The lipophilicity of a series of anticancer propargylquinoline derivatives is investigated using both chromatographic and computational methods. The parameters of the tested compounds' relative lipophilicity (logkw) are determined experimentally by the high-performance liquid chromatographic method (RP-HPLC, Accucore C18 column), using
D R Boyd et al.
Organic & biomolecular chemistry, 8(5), 1081-1090 (2010-02-19)
A series of enantiopure 2,2'-bipyridines have been synthesised from the corresponding cis-dihydrodiol metabolites of 2-chloroquinolines. Several of the resulting hydroxylated 2,2'-bipyridines were found to be useful chiral ligands for the asymmetric aminolysis of meso-epoxides leading to the formation of enantioenriched
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.