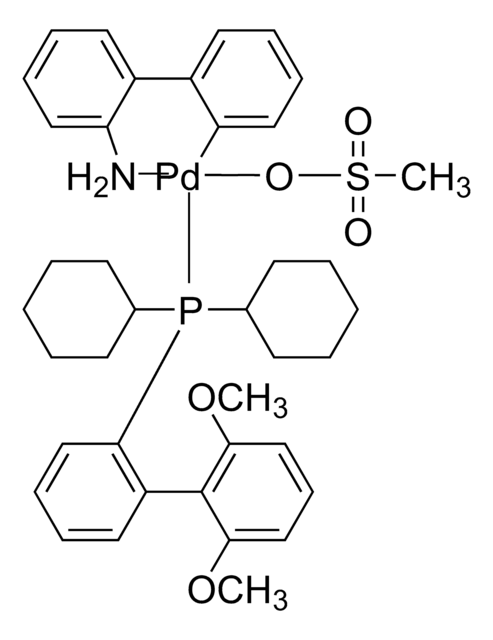
912476
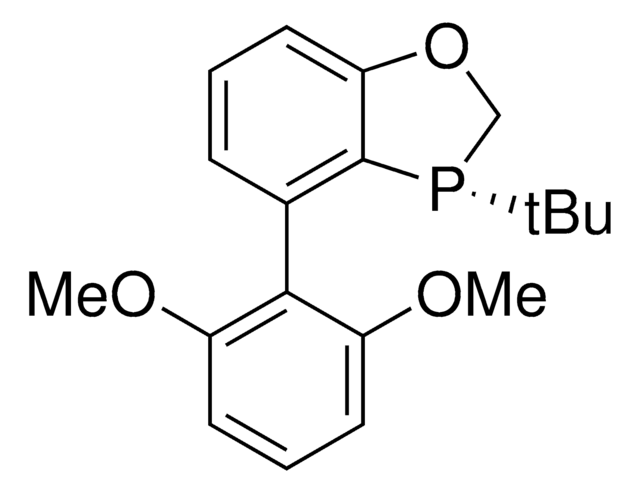
(S)-AntPhos
≥97%
Sinonimo/i:
(S)-4-(Anthracen-9-yl)-3-(tert-butyl)-2,3-dihydrobenzo[d][1,3]oxaphosphole
Autenticatiper visualizzare i prezzi riservati alla tua organizzazione & contrattuali
About This Item
Formula empirica (notazione di Hill):
C25H23OP
Numero CAS:
Peso molecolare:
370.42
Codice UNSPSC:
12352200
NACRES:
NA.22
Prodotti consigliati
Livello qualitativo
Saggio
≥97%
Forma fisica
powder
Purezza ottica
ee: ≥99% (HPLC)
Impiego in reazioni chimiche
reagent type: ligand
Gruppo funzionale
phosphine
Applicazioni
(S)-AntPhos is a P-chiral monophosphorus ligand used for the asymmetric Suzuki-Miyaura and Miyaura borylation reactions. This ligand is uniquely effective for sterically hindered cross-coupling reactions.
Note legali
Sold in collaboration with Zejun Pharmaceuticals
Prodotti correlati
N° Catalogo
Descrizione
Determinazione del prezzo
Codice della classe di stoccaggio
11 - Combustible Solids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Scegli una delle versioni più recenti:
Certificati d'analisi (COA)
Lot/Batch Number
Non trovi la versione di tuo interesse?
Se hai bisogno di una versione specifica, puoi cercare il certificato tramite il numero di lotto.
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
Wenzhen Fu et al.
Angewandte Chemie (International ed. in English), 54(8), 2520-2524 (2015-01-20)
The first asymmetric nickel-catalyzed intramolecular reductive cyclization of alkynones is reported. A P-chiral monophosphine and triethylsilane were used as the ligand and the reducing reagent, respectively, to form a series of tertiary allylic alcohols bearing furan/pyran rings in excellent yields
Naifu Hu et al.
Angewandte Chemie (International ed. in English), 55(16), 5044-5048 (2016-03-19)
A highly enantioselective alkene aryloxyarylation led to the high-yielding formation of a series of 1,4-benzodioxanes, 1,4-benzooxazines, and chromans containing quaternary stereocenters with excellent enantioselectivity. The sterically bulky and conformationally well defined chiral monophosphorus ligand L4 or L5 was responsible for
Ruofei Cheng et al.
Journal of the American Chemical Society, 140(13), 4508-4511 (2018-03-27)
Carborane cage chirality is an outstanding issue of great interest as the icosahedral carboranes have wide applications in medicinal and materials chemistry. The synthesis of optically active carborane derivatives, whose chirality is associated with the substitution patterns on the polyhedron
Naifu Hu et al.
Journal of the American Chemical Society, 137(21), 6746-6749 (2015-05-06)
The rhodium-catalyzed asymmetric hydroboration of α-arylenamides with BI-DIME as the chiral ligand and (Bpin)2 as the reagent yields for the first time a series of α-amino tertiary boronic esters in good yields and excellent enantioselectivities (up to 99% ee).
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.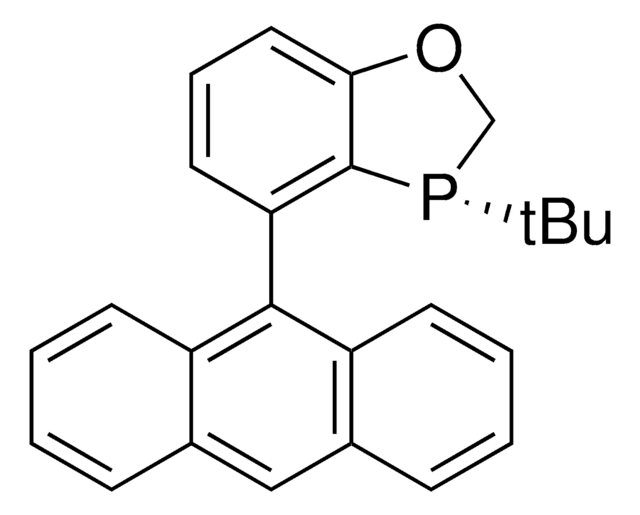
![(R)-1-[(SP)-2-(Diphenylphosphino)ferrocenyl]ethyldicyclohexylphosphine ≥97%](/deepweb/assets/sigmaaldrich/product/structures/245/493/2ae2dd8a-65cc-4aba-9a1f-1292eb1ad8e0/640/2ae2dd8a-65cc-4aba-9a1f-1292eb1ad8e0.png)