75490
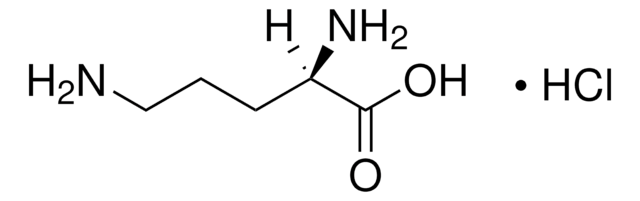
DL-Ornithine monohydrochloride
≥99.0% (AT)
Sinonimo/i:
(±)-2,5-Diaminopentanoic acid monohydrochloride
Autenticatiper visualizzare i prezzi riservati alla tua organizzazione & contrattuali
About This Item
Formula condensata:
NH2(CH2)3CH(CO2H)(NH2) · HCl
Numero CAS:
Peso molecolare:
168.62
Beilstein:
4153338
Numero CE:
Numero MDL:
Codice UNSPSC:
12352209
ID PubChem:
NACRES:
NA.22
Prodotti consigliati
Livello qualitativo
Saggio
≥99.0% (AT)
Stato
powder
Impiego in reazioni chimiche
reaction type: solution phase peptide synthesis
Punto di fusione
~233 °C (dec.)
applicazioni
peptide synthesis
Stringa SMILE
Cl.NCCCC(N)C(O)=O
InChI
1S/C5H12N2O2.ClH/c6-3-1-2-4(7)5(8)9;/h4H,1-3,6-7H2,(H,8,9);1H
GGTYBZJRPHEQDG-UHFFFAOYSA-N
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Applicazioni
DL-Ornithine monohydrochloride may be used as a starting material in the synthesis of (-)-(1-2H)putrescine dihydrochloride via enzymatic decarboxylation.
Codice della classe di stoccaggio
11 - Combustible Solids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Dispositivi di protezione individuale
Eyeshields, Gloves, type N95 (US)
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
The Stereochemistry of the Enzymic Decarboxylation of l-Arginine and of l-Ornithine
Richards JC and Spenser ID
Canadian Journal of Chemistry, 60(22), 2810-2820 (1982)
Juan C Marini et al.
American journal of physiology. Endocrinology and metabolism, 303(11), E1348-E1353 (2012-10-18)
Citrulline is an amino acid synthesized in the gut and utilized for the synthesis of the conditionally essential amino acid arginine. Recently, the origin of the ornithine utilized for citrulline synthesis has become a matter of discussion. Multiple physiological factors
Sherry Dadsetan et al.
Biochemical pharmacology, 85(1), 115-123 (2012-10-30)
Combined administration of ornithine and phenylacetate (OP) is proposed as a novel treatment of hyperammonemia and hepatic encephalopathy. Ornithine is believed to increase ammonia fixation into glutamine in muscle tissue and glutamine is subsequently thought to react with phenylacetate forming
Nicole Berthold et al.
Antimicrobial agents and chemotherapy, 57(1), 402-409 (2012-11-02)
Proline-rich antimicrobial peptides (PrAMPs) from insects and mammals have recently been evaluated for their pharmaceutical potential in treating systemic bacterial infections. Besides the native peptides, several shortened, modified, or even artificial sequences were highly effective in different murine infection models.
D Amelio et al.
Comparative biochemistry and physiology. Part A, Molecular & integrative physiology, 164(2), 356-362 (2012-11-06)
The Frank-Starling law is a fundamental property of the vertebrate myocardium which allows, when the end-diastolic volume increases, that the consequent stretch of the myocardial fibers generates a more forceful contraction. It has been shown that in the eel (Anguilla
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.