710776
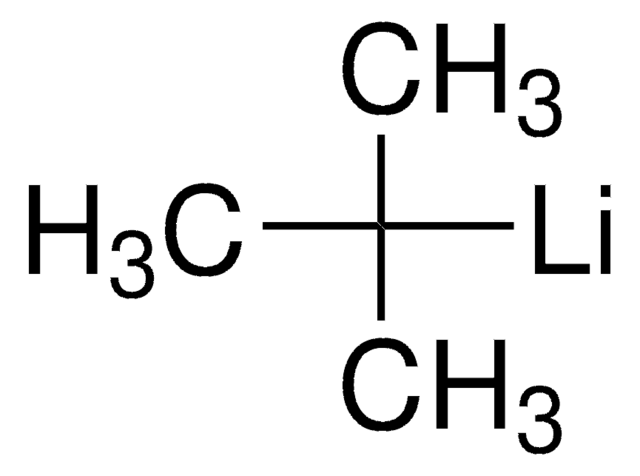
n-Butillitio
1.4 M in toluene
Sinonimo/i:
n-BuLi, Butillitio, Litio-1-butanide
Autenticatiper visualizzare i prezzi riservati alla tua organizzazione & contrattuali
About This Item
Formula condensata:
CH3(CH2)3Li
Numero CAS:
Peso molecolare:
64.06
Beilstein:
1209227
Numero MDL:
Codice UNSPSC:
12352103
ID PubChem:
NACRES:
NA.22
Prodotti consigliati
Forma fisica
liquid
Concentrazione
1.4 M in toluene
Densità
0.849 g/mL at 25 °C
Temperatura di conservazione
2-8°C
Stringa SMILE
[Li]CCCC
InChI
1S/C4H9.Li/c1-3-4-2;/h1,3-4H2,2H3;
MZRVEZGGRBJDDB-UHFFFAOYSA-N
Avvertenze
Danger
Indicazioni di pericolo
Classi di pericolo
Aquatic Chronic 3 - Asp. Tox. 1 - Eye Dam. 1 - Flam. Liq. 2 - Pyr. Liq. 1 - Repr. 2 - Skin Corr. 1B - STOT RE 2 Inhalation - STOT SE 3 - Water-react 1
Organi bersaglio
Central nervous system
Rischi supp
Codice della classe di stoccaggio
4.2 - Pyrophoric and self-heating hazardous materials
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
21.2 °F
Punto d’infiammabilità (°C)
-6 °C
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
Yuqiang Ma et al.
ACS nano, 9(7), 7383-7391 (2015-07-01)
Two-dimensional (2D) semiconducting monolayer transition metal dichalcogenides (TMDCs) have stimulated lots of interest because they are direct bandgap materials that have reasonably good mobility values. However, contact between most metals and semiconducting TMDCs like 2H phase WSe2 are highly resistive
Michael A Tarselli et al.
Organic letters, 11(20), 4596-4599 (2009-10-09)
A procedure for the coupling of aliphatic imines with allylic and allenic alkoxides is described. The success of these studies was enabled by a unique reactivity profile of Ti(IV) isopropoxide/n-BuLi compared to well-known Ti(IV) isopropoxide/RMgX systems.
J P Parente et al.
Carbohydrate research, 141(1), 41-47 (1985-08-15)
Treatment of dimethyl sulfoxide with butyllithium leads to rapid formation of lithium methylsulfinyl carbanion. The reaction products tend to be significantly freer from impurities when lithium methylsulfinyl carbanion is used rather than sodium or potassium methylsulfinyl carbanion. This reagent gives
Lawrence M Pratt et al.
The Journal of organic chemistry, 68(16), 6387-6391 (2003-08-05)
The effects of lithium dialkylamide structure, mixed aggregate formation, and solvation on the stereoselectivity of ketone enolization were examined. Of the lithium dialkylamides examined, lithium tetramethylpiperidide (LiTMP) in THF resulted in the best enolization selectivity. The stereoselectivity was further improved
Graeme Barker et al.
Organic letters, 12(18), 4176-4179 (2010-08-20)
A diamine-free protocol for the s-BuLi-mediated lithiation-trapping of N-Boc heterocycles has been developed. In the optimized procedure, lithiation is accomplished using s-BuLi in THF at -30 °C for only 5 or 10 min. Subsequent electrophilic trapping or transmetalation-Negishi coupling delivered
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.