52985
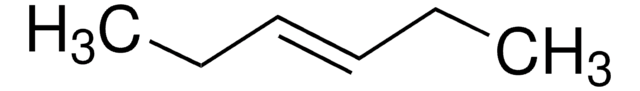
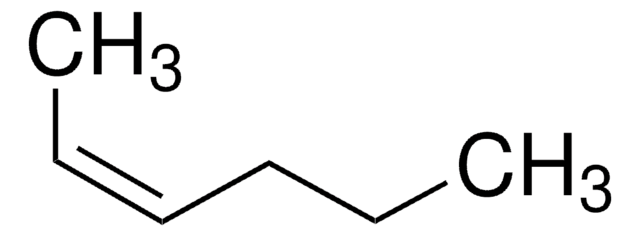
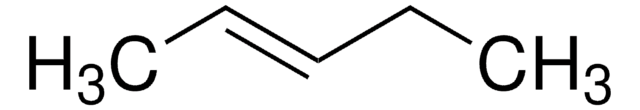
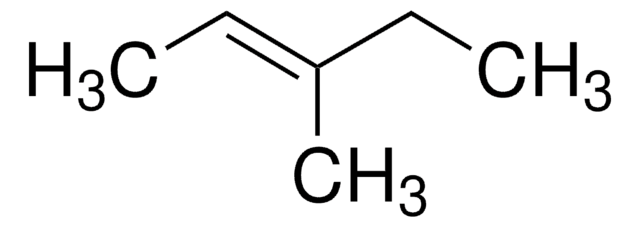
cis-3-Hexene
≥95.0%
Autenticatiper visualizzare i prezzi riservati alla tua organizzazione & contrattuali
About This Item
Formula empirica (notazione di Hill):
C6H12
Numero CAS:
Peso molecolare:
84.16
Beilstein:
1718858
Numero MDL:
Codice UNSPSC:
12352100
ID PubChem:
NACRES:
NA.22
Prodotti consigliati
Livello qualitativo
Saggio
≥95.0%
Indice di rifrazione
n20/D 1.395
P. ebollizione
66-68 °C (lit.)
Densità
0.681 g/mL at 20 °C (lit.)
Stringa SMILE
CC\C=C/CC
InChI
1S/C6H12/c1-3-5-6-4-2/h5-6H,3-4H2,1-2H3/b6-5-
ZQDPJFUHLCOCRG-WAYWQWQTSA-N
Descrizione generale
cis-3-Hexene is a symmetrical cis-disubstituted alkene that can be prepared by the hydroboration of 3-hexyne followed by protonolysis. The gas-phase study of its molecular structure by electron diffraction combined with molecular mechanical calculations reveals the presence of the (+ac, +ac) and the (-ac, +ac) forms. cis-3-Hexene undergoes epoxidation with dimethyldioxirane to form the corresponding epoxide.
Applicazioni
cis-3-Hexene may be used in the preparation of 3-hexanol via asymmetric hydroboration with diisopinocampheylborane (Ipc2BH).
Avvertenze
Danger
Indicazioni di pericolo
Consigli di prudenza
Classi di pericolo
Asp. Tox. 1 - Eye Irrit. 2 - Flam. Liq. 2
Codice della classe di stoccaggio
3 - Flammable liquids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
-13.0 °F - closed cup
Punto d’infiammabilità (°C)
-25 °C - closed cup
Dispositivi di protezione individuale
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
Epoxidation by dimethyldioxirane. Electronic and steric effects.
Baumstark AL and Vasquez PC.
The Journal of Organic Chemistry, 53(15), 3437-3439 (1988)
Hydroboration. XI. The hydroboration of acetylenes-A convenient conversion of internal acetylenes into cis-olefins and of terminal acetylenes into aldehydes
Brown HC and Zweifel G.
Journal of the American Chemical Society, 83(18), 3834-3840 (1961)
Hydroboration. 61. Diisopinocampheylborane of high optical purity. Improved preparation and asymmetric hydroboration of representative cis-disubstituted alkenes.
Brown HC, et al.
The Journal of Organic Chemistry, 47(26), 5065-5069 (1982)
The molecular structures of cis-3-hexene and trans-3-hexene in the gas phase by electron diffraction and molecular mechanical calculations.
Van HD, et al.
Journal of Molecular Structure, 74(1), 123-135 (1981)
Nadhem Aissani et al.
Journal of agricultural and food chemistry, 63(27), 6120-6125 (2015-06-18)
Research on new pesticides based on plant extracts, aimed at the development of nontoxic formulates, has recently gained increased interest. This study investigated the use of the volatilome of rucola (Eruca sativa) as a powerful natural nematicidal agent against the
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.