471801
4-Pentenoic anhydride
98%
Autenticatiper visualizzare i prezzi riservati alla tua organizzazione & contrattuali
About This Item
Formula condensata:
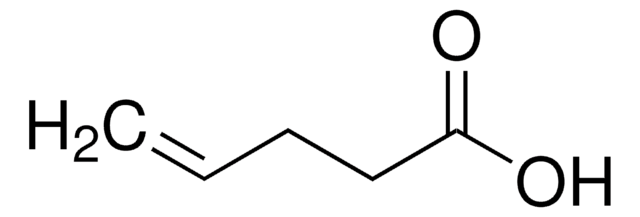
(H2C=CHCH2CH2CO)2O
Numero CAS:
Peso molecolare:
182.22
Numero MDL:
Codice UNSPSC:
12352100
ID PubChem:
NACRES:
NA.22
Prodotti consigliati
Saggio
98%
Indice di rifrazione
n20/D 1.447 (lit.)
P. ebollizione
78-81 °C/0.4 mmHg (lit.)
Densità
0.997 g/mL at 25 °C (lit.)
Gruppo funzionale
allyl
anhydride
ester
Stringa SMILE
C=CCCC(=O)OC(=O)CCC=C
InChI
1S/C10H14O3/c1-3-5-7-9(11)13-10(12)8-6-4-2/h3-4H,1-2,5-8H2
NEDHQDYBHYNBIF-UHFFFAOYSA-N
Categorie correlate
Descrizione generale
4-Pentenoic anhydride is a carboxylic anhydride.
Applicazioni
4-Pentenoic anhydride may be used:
- In the preparation of glucose functionalized (co)polymers.
- As a monomer in the preparation of cross-linked polyanhydrides.
- For the preparation of polymers with pendant vinyl or acetylene.
Avvertenze
Warning
Indicazioni di pericolo
Consigli di prudenza
Classi di pericolo
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Organi bersaglio
Respiratory system
Codice della classe di stoccaggio
10 - Combustible liquids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
230.0 °F - closed cup
Punto d’infiammabilità (°C)
110 °C - closed cup
Dispositivi di protezione individuale
Eyeshields, Gloves, type ABEK (EN14387) respirator filter
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
2-(Trimethylsilyl) ethyl Glycosides. Transformation into the Corresponding 1-O-Acyl Sugars.
Ellervik U and Magnusson G.
Acta Chemica Scandinavica, 47, 826-826 (1993)
Studies of the mechanism of the hypoglycemic action of 4-pentenoic acid.
Corredor C, et al.
Proceedings of the National Academy of Sciences of the USA, 58(6), 2299-2299 (1967)
Gaojian Chen et al.
Chemical communications (Cambridge, England), (10)(10), 1198-1200 (2009-02-26)
Homopolymer and block copolymer bearing carbohydrate side chain functionality were obtained by grafting glucothiose onto alkene functional scaffolds via a thiol-ene click reaction and the resulting copolymer was used to form thermo-responsive micelles as a potential drug carrier.
Katie L Poetz et al.
Biomacromolecules, 15(7), 2573-2582 (2014-05-23)
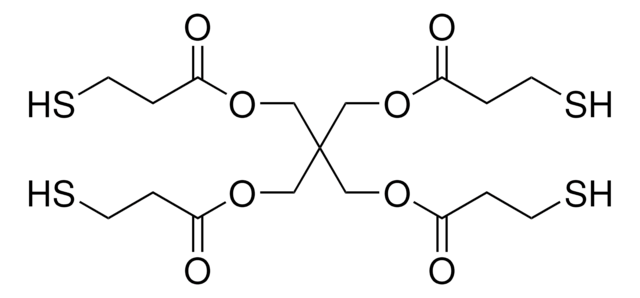
Several critical aspects of cross-linked polyanhydrides made using thiol-ene polymerization are reported, in particular the erosion, release, and solution properties, along with their cytotoxicity toward fibroblast cells. The monomers used to synthesize these polyanhydrides were 4-pentenoic anhydride and pentaerythritol tetrakis(3-mercaptopropionate).
Vien T Huynh et al.
Biomacromolecules, 12(5), 1738-1751 (2011-04-12)
Statistical and block copolymers based on poly(2-hydroxyethyl methacrylate) (PHEMA) and poly[oligo(ethylene glycol) methylether methacrylate] (POEGMEMA) were modified with 4-pentenoic anhydride or 4-oxo-4-(prop-2-ynyloxy)butanoic anhydride to generate polymers with pendant vinyl or acetylene, respectively. Subsequent thiol-ene or thiol-yne reaction with thioglycolic acid
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.