426369
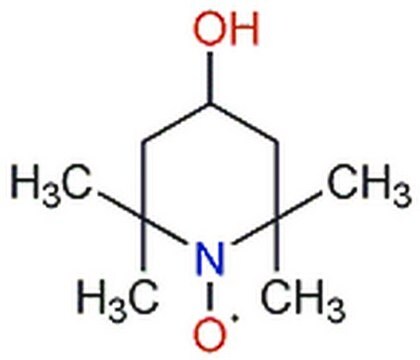
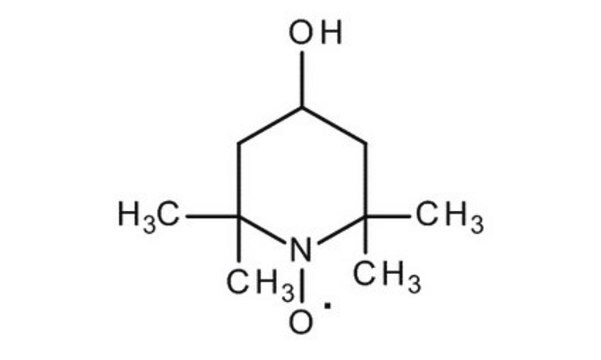
TEMPO
purified by sublimation, 99%
Sinonimo/i:
2,2,6,6-Tetramethylpiperidine 1-oxyl, 2,2,6,6-Tetramethyl-1-piperidinyloxy, free radical, TEMPO
About This Item
Prodotti consigliati
Livello qualitativo
Saggio
99%
Stato
solid
Purificato mediante
sublimation
Impiego in reazioni chimiche
reagent type: oxidant
Punto di fusione
36-38 °C (lit.)
Temperatura di conservazione
2-8°C
Stringa SMILE
CC1(C)CCCC(C)(C)N1[O]
InChI
1S/C9H18NO/c1-8(2)6-5-7-9(3,4)10(8)11/h5-7H2,1-4H3
QYTDEUPAUMOIOP-UHFFFAOYSA-N
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Descrizione generale
Applicazioni
Avvertenze
Danger
Indicazioni di pericolo
Classi di pericolo
Aquatic Chronic 3 - Eye Dam. 1 - Skin Corr. 1C
Codice della classe di stoccaggio
8A - Combustible corrosive hazardous materials
Classe di pericolosità dell'acqua (WGK)
WGK 2
Punto d’infiammabilità (°F)
152.6 °F - closed cup
Punto d’infiammabilità (°C)
67 °C - closed cup
Dispositivi di protezione individuale
Eyeshields, Faceshields, Gloves, type P3 (EN 143) respirator cartridges
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
Articoli
Block Copolymer Synthesis Using a Nitroxide-mediated Radical Polymerization (NMP) Approach
Block copolymer synthesis using a commercially available nitroxide-mediated radical polymerization (NMP) initiator
TEMPO (2,2,6,6-Tetramethylpiperidinyloxy or 2,2,6,6-Tetramethylpiperidine 1-oxyl) and its derivatives are stable nitroxy radicals used as catalysts in organic oxidation reactions. TEMPO was discovered by Lebedev and Kazarnovskii in 1960. The stable free radical nature of TEMPO is due to the presence of bulky substituent groups, which hinder the reaction of the free radical with other molecules.
We presents an article about a micro review of reversible addition/fragmentation chain transfer (RAFT) polymerization. RAFT (Reversible Addition/Fragmentation Chain Transfer) polymerization is a reversible deactivation radical polymerization (RDRP) and one of the more versatile methods for providing living characteristics to radical polymerization.
Protocolli
Sigma-Aldrich presents an article about RAFT, or Reversible Addition/Fragmentation Chain Transfer, which is a form of living radical polymerization.
We presents an article featuring procedures that describe polymerization of methyl methacrylate and vinyl acetate homopolymers and a block copolymer as performed by researchers at CSIRO.
Sigma-Aldrich presents an article about the typical procedures for polymerizing via ATRP, which demonstrates that in the following two procedures describe two ATRP polymerization reactions as performed by Prof. Dave Hadddleton′s research group at the University of Warwick.
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.