378151
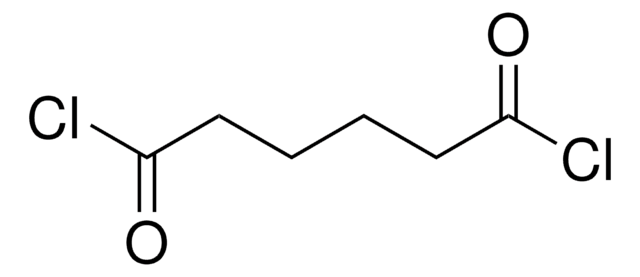
Diglycolyl chloride
95%
Sinonimo/i:
2,2′-Oxydiacetyl chloride
Autenticatiper visualizzare i prezzi riservati alla tua organizzazione & contrattuali
About This Item
Formula condensata:
O(CH2COCl)2
Numero CAS:
Peso molecolare:
170.98
Numero CE:
Numero MDL:
Codice UNSPSC:
12352100
ID PubChem:
NACRES:
NA.22
Prodotti consigliati
Livello qualitativo
Saggio
95%
Stato
liquid
Indice di rifrazione
n20/D 1.473 (lit.)
P. ebollizione
84-87 °C/2 mmHg (lit.)
Densità
1.439 g/mL at 25 °C (lit.)
Gruppo funzionale
acyl chloride
ether
Stringa SMILE
ClC(=O)COCC(Cl)=O
InChI
1S/C4H4Cl2O3/c5-3(7)1-9-2-4(6)8/h1-2H2
GTZXSBQCNBNWPK-UHFFFAOYSA-N
Categorie correlate
Descrizione generale
Diglycolyl chloride (2,2′-Oxydiacetyl chloride) is an acid halide.
Applicazioni
Diglycolyl chloride is suitable for use in the synthesis of ply(ether ester). It may be used in the synthesis of:
- chiral diphenyl substituted polyether-diester compounds
- morpholine dione analog (IMDNQ)
- salicylic acid (SA)- based diacids
Diglycolyl chloride may be used in the synthesis of the following compounds:
- diazadibenzo-18-crown-6 diamide
- diazadi(tert-butylbenzo)-18-crown-6 diamide
- surfen derivative
Avvertenze
Danger
Indicazioni di pericolo
Classi di pericolo
Eye Dam. 1 - Skin Corr. 1B
Codice della classe di stoccaggio
8A - Combustible corrosive hazardous materials
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
235.4 °F - closed cup
Punto d’infiammabilità (°C)
113 °C - closed cup
Dispositivi di protezione individuale
Faceshields, Gloves, Goggles, type ABEK (EN14387) respirator filter
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
Preparation of chiral diphenyl substituted polyether-diester compounds.
Bradshaw JS, et al.
The Journal of Organic Chemistry, 47(7), 1229-1232 (1982)
Ashley L Carbone et al.
Macromolecular rapid communications, 30(12), 1021-1021 (2010-02-18)
Fast-degrading, salicylate-based poly(anhydride-esters) were designed to degrade and release the active component, salicylic acid (SA), within 1 week. The polymer degradation was enhanced by using shorter or oxygen-containing aliphatic chains. A copolymer of diglycolic acid was also made with a
Synthesis and CO2 Solubility Studies of Poly (ether carbonate) s and Poly (ether ester) s Produced by Step Growth Polymerization.
Tan B, et al.
Macromolecules, 38(5), 1691-1698 (2005)
J C Aguilar et al.
Talanta, 54(6), 1195-1204 (2008-10-31)
The ligands 4,7-diaza-2,3,8,9-dibenzo-15-crown-5 (L1), 4,10-diaza-2,3,11,12-dibenzo-18-crown-6 (L2), 4,10-diaza-2,3,11,12-di(4'-tert-butylbenzo)-18-crown-6 (L3) and N,N-di(methylenecarboxyethoxy) 4,10-diaza-2,3,11,12-dibenzo-18-crown-6 (L4) have been prepared. Partition coefficients and acid dissociation constants for these four diazadibenzocrown ether compounds were determined in water-chloroform. Their effectiveness was assessed in solvent extraction of Pb(2+)
Small molecule antagonists of cell-surface heparan sulfate and heparin-protein interactions.
Weiss RJ, et al.
Chemical Science, 6(10), 5984-5993 (2015)
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.