281077
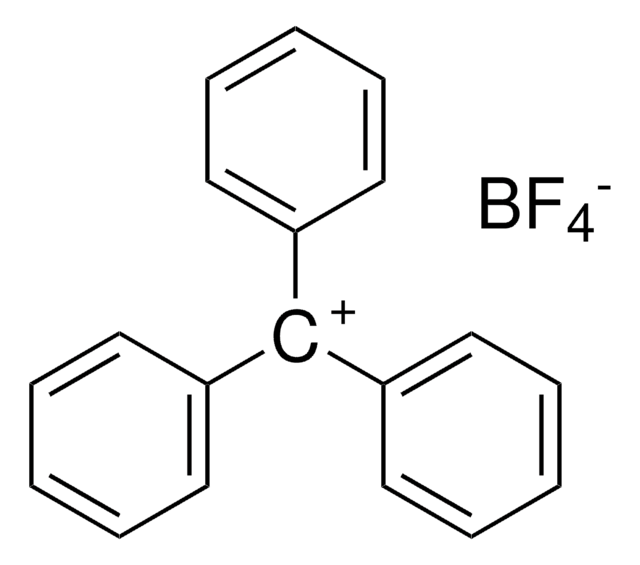
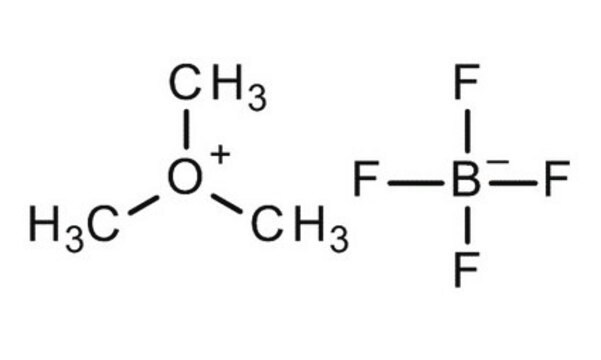
Trimethyloxonium tetrafluoroborate
95%
Sinonimo/i:
Trimethyloxonium fluoroborate
Autenticatiper visualizzare i prezzi riservati alla tua organizzazione & contrattuali
About This Item
Formula condensata:
(CH3)3O(BF4)
Numero CAS:
Peso molecolare:
147.91
Beilstein:
3597303
Numero CE:
Numero MDL:
Codice UNSPSC:
12352107
ID PubChem:
NACRES:
NA.22
Prodotti consigliati
Livello qualitativo
Saggio
95%
Stato
solid
Gruppo funzionale
ether
Temperatura di conservazione
−20°C
Stringa SMILE
C[O+](C)C.F[B-](F)(F)F
InChI
1S/C3H9O.BF4/c1-4(2)3;2-1(3,4)5/h1-3H3;/q+1;-1
CZVZBKHWOFJNCR-UHFFFAOYSA-N
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Descrizione generale
Trimethyloxonium tetrafluoroborate can be used as a methylating agent for the methylation of hydroxyl/carboxyl functional groups. It is capable of methylating polyfunctional carboxylic acids. It is also used as a catalyst for the polymerization of cyclic sulfides and ethers.
Applicazioni
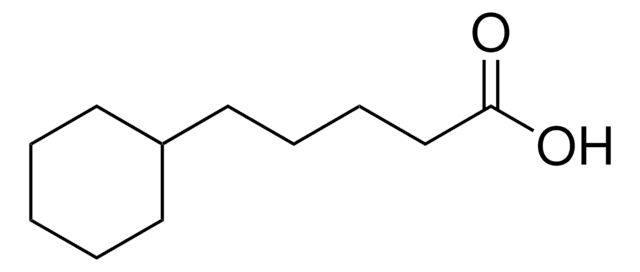
Reagent for the methylation of hydroxyl groups recently used in a complex, multistep synthesis directed towards spirastrellolide, a marine natural product.
Avvertenze
Danger
Indicazioni di pericolo
Classi di pericolo
Skin Corr. 1B
Rischi supp
Codice della classe di stoccaggio
8A - Combustible corrosive hazardous materials
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Dispositivi di protezione individuale
Eyeshields, Faceshields, Gloves, type P3 (EN 143) respirator cartridges
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
Toward the total synthesis of spirastrellolide A. Part 2: Conquest of the northern hemisphere.
Alois Fürstner et al.
Angewandte Chemie (International ed. in English), 45(33), 5510-5515 (2006-08-15)
H M Liebich et al.
Journal of chromatography. A, 843(1-2), 237-245 (1999-07-10)
Trimethyloxonium tetrafluoroborate (TMO) is applied as derivatising reagent to transform urinary organic acids into their methyl esters. The method is suggested as an alternative to the use of diazomethane which is carcinogenic and explosive. In contrast to other methods avoiding
Marco Pacenti et al.
Biomedical chromatography : BMC, 22(10), 1155-1163 (2008-05-29)
A method for the determination of the organic acids directly in the urine employing derivatization with trimethyloxonium tetrafluoroborate as a methylating agent and sequential extraction by head space and direct immersion/solid phase microextraction is reported. Furoic acid, hippuric acid, methylhippuric
S Chericoni et al.
Journal of analytical toxicology, 35(4), 193-198 (2011-04-26)
The present work describes the validation of a novel aqueous in situ derivatization procedure with trimethyloxonium tetrafluoroborate (TMO) as methylating agent for the simultaneous, quantitative analysis of Δ(9)-tetrahydrocannabinol (THC) and 11-nor-Δ(9)-tetrahydrocannabinol carboxylic acid (THC-COOH) in human urine. The derivatizing agent
H M Liebich et al.
Journal of chromatography. B, Biomedical sciences and applications, 713(2), 427-432 (1998-09-24)
We developed a new sample preparation method for profiling organic acids in urine by GC or GC-MS. The method includes derivatisation of the organic acids directly in the aqueous urine using trimethyloxonium tetrafluoroborate as a methylating agent, extraction of the
Global Trade Item Number
| SKU | GTIN |
|---|---|
| 281077-100G | 4065265906913 |
| 281077-10G | 4061826214626 |
| 281077-1G | 4061826671436 |
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.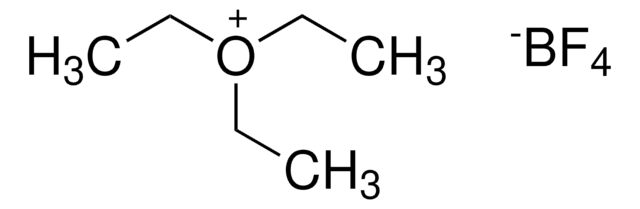


![1,8-diazabiciclo[5.4.0]undec-7-ene 98%](/deepweb/assets/sigmaaldrich/product/structures/120/564/5b373e23-1624-489c-8efb-692de0f96ffb/640/5b373e23-1624-489c-8efb-692de0f96ffb.png)