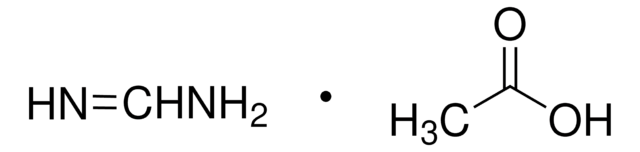268607
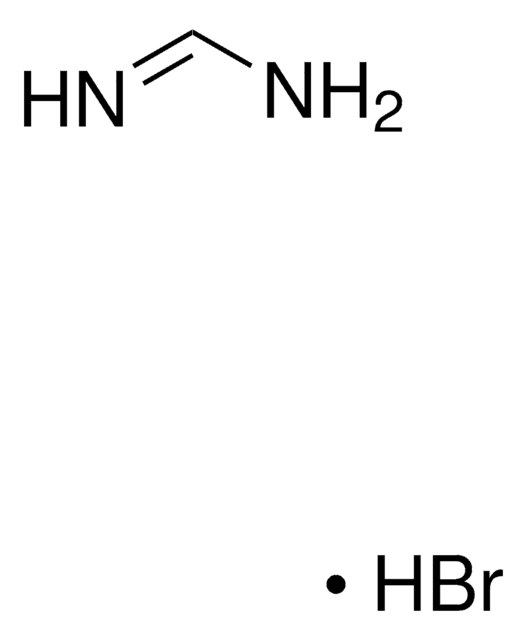
Formamidine hydrochloride
97%
Autenticatiper visualizzare i prezzi riservati alla tua organizzazione & contrattuali
About This Item
Formula condensata:
HN=CHNH2 · HCl
Numero CAS:
Peso molecolare:
80.52
Beilstein:
3906935
Numero CE:
Numero MDL:
Codice UNSPSC:
12352100
ID PubChem:
NACRES:
NA.22
Prodotti consigliati
Livello qualitativo
Saggio
97%
Stato
solid
Punto di fusione
84-87 °C (lit.)
Gruppo funzionale
amine
Stringa SMILE
Cl[H].[H]C(N)=N
InChI
1S/CH4N2.ClH/c2-1-3;/h1H,(H3,2,3);1H
NMVVJCLUYUWBSZ-UHFFFAOYSA-N
Categorie correlate
Applicazioni
Formamidine hydrochloride was used in the synthesis of imidazoleglycerol phosphate (IGP). It was also used in the synthesis of 5-methyl-4,6-dihydroxypyrimidine.
Codice della classe di stoccaggio
11 - Combustible Solids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Dispositivi di protezione individuale
Eyeshields, Gloves, type N95 (US)
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
The biosynthesis of histidine; D-erythro-imidazoleglycerol phosphate dehydrase.
B N AMES
The Journal of biological chemistry, 228(1), 131-143 (1957-09-01)
Reactions of oxidizing radicals with 4, 6-dihydroxypyrimidines as model compounds for uracil, thymine, and cytosine.
The Journal of Physical Chemistry, 91(2), 426-433 (1987)
Federico Bertasi et al.
Physical chemistry chemical physics : PCCP, 19(38), 26230-26239 (2017-09-22)
This work describes the preparation of the new lipophilic ionic liquid tetraoctyl-formamidinium bis(trifluoromethanesulfonyl) imide (TOFATFSI), which is miscible with lower alkanes. In particular, this work focuses on the electric behaviour of TOFATFSI in the particularly challenging highly apolar environment of
José M Casas et al.
Inorganic chemistry, 44(25), 9444-9452 (2005-12-06)
The preparation of the [NBu4][Pt(C6F5)3L] complexes (L=triazene, formamidine, 2-aminopyridine,) have been carried out. These ligands contain a hydrogen atom, with more or less acidic character, in a position suitable for establishing an intramolecular hydrogen bonding interaction with the metal center.
Carsten Präsang et al.
Journal of the American Chemical Society, 127(29), 10182-10183 (2005-07-21)
Carbene analogues of borazines are highly thermally stable. Keeping quasi-identical steric demands, the electronic properties of the carbene can be precisely tuned by varying the nature of the substituents at the boron centers.
Global Trade Item Number
| SKU | GTIN |
|---|---|
| 268607-5G | 4061826134788 |
| 268607-25G | 4061826134771 |
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.