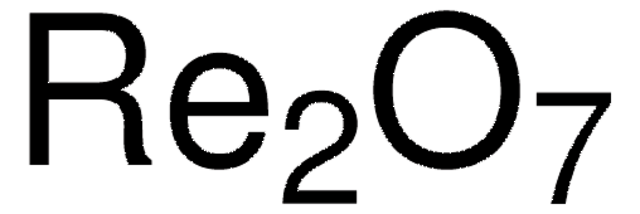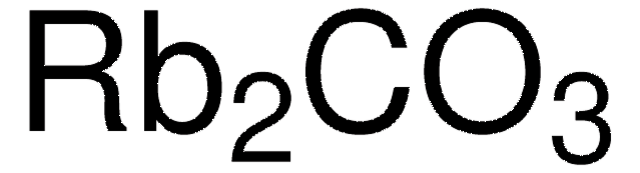267317
Rhenium
foil, thickness 0.25 mm, 99.98% trace metals basis
Sinonimo/i:
Rhenium element
About This Item
Prodotti consigliati
Livello qualitativo
Saggio
99.98% trace metals basis
Stato
foil
Descrizione
19.3 μΩ-cm, 20°C
Spessore
0.25 mm
P. ebollizione
5596 °C (lit.)
5627 °C (lit.)
Punto di fusione
3180 °C (lit.)
Densità
21.02 g/cm3 (lit.)
Stringa SMILE
[ReH]
InChI
1S/Re
WUAPFZMCVAUBPE-UHFFFAOYSA-N
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Descrizione generale
Applicazioni
It can be used as an additive to prepare a molybdenum-titanium-zirconium (TZM) alloy joint to improve its tensile strength.
It can also be used as a catalyst for various hydrodeoxygenation reactions.
Quantità
Codice della classe di stoccaggio
13 - Non Combustible Solids
Classe di pericolosità dell'acqua (WGK)
nwg
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Dispositivi di protezione individuale
Eyeshields, Gloves, type P3 (EN 143) respirator cartridges
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
Articoli
Can there be an effective strategy for finding breakthrough materials, since they are, by definition, unpredictable? One answer is found in Combinatorial Materials Science techniques, which represent a powerful approach to identifying new and unexpected materials.
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.