241636
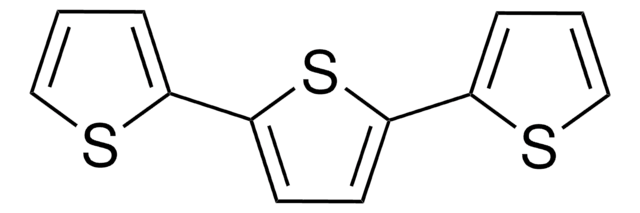
2,2′-Bithiophene
99%
Sinonimo/i:
2,2′-Bithienyl, 2,2′-Dithienyl
About This Item
Prodotti consigliati
Saggio
99%
P. eboll.
260 °C (lit.)
Punto di fusione
32-33 °C (lit.)
Stringa SMILE
c1csc(c1)-c2cccs2
InChI
1S/C8H6S2/c1-3-7(9-5-1)8-4-2-6-10-8/h1-6H
OHZAHWOAMVVGEL-UHFFFAOYSA-N
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Descrizione generale
Applicazioni
Codice della classe di stoccaggio
10 - Combustible liquids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
230.0 °F - closed cup
Punto d’infiammabilità (°C)
110 °C - closed cup
Dispositivi di protezione individuale
Eyeshields, Gloves, multi-purpose combination respirator cartridge (US)
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
Articoli
From Form to Function: Molding Porous Materials in Three Dimensions by Colloidal Crystal Templating
Professor Rivnay (Northwestern University, USA) discusses using organic mixed conductors as an alternative to efficiently bridge the ionic world of biology with contemporary microelectronics.
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.
![Thieno[3,2-b]thiophene 95%](/deepweb/assets/sigmaaldrich/product/structures/353/609/429fd4bf-e217-4371-80a3-9e5a4d88908b/640/429fd4bf-e217-4371-80a3-9e5a4d88908b.png)
![2,5-Bis(trimethylstannyl)-thieno[3,2-b]thiophene 97%](/deepweb/assets/sigmaaldrich/product/structures/126/532/26557e94-858e-4c96-90de-ca88d84a8727/640/26557e94-858e-4c96-90de-ca88d84a8727.png)
![Benzo[1,2-b:4,5-b′]dithiophene-4,8-dione 97%](/deepweb/assets/sigmaaldrich/product/structures/418/544/b7faac0b-ad09-4b42-a9fa-aeb38017a39e/640/b7faac0b-ad09-4b42-a9fa-aeb38017a39e.png)