168521
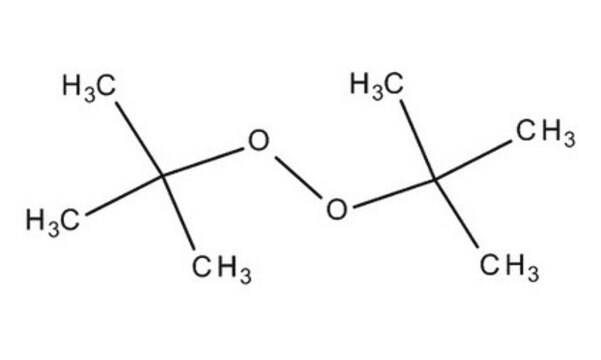
Luperox® DI, tert-Butyl peroxide
98%
Sinonimo/i:
tert-Butyl peroxide, Di-tert-butyl peroxide
About This Item
Prodotti consigliati
Tensione di vapore
40 mmHg ( 20 °C)
Livello qualitativo
Saggio
98%
Forma fisica
liquid
Impiego in reazioni chimiche
reagent type: oxidant
Indice di rifrazione
n20/D 1.3891 (lit.)
P. eboll.
109-110 °C (lit.)
Densità
0.796 g/mL at 25 °C (lit.)
Temperatura di conservazione
2-8°C
Stringa SMILE
CC(C)(C)OOC(C)(C)C
InChI
1S/C8H18O2/c1-7(2,3)9-10-8(4,5)6/h1-6H3
LSXWFXONGKSEMY-UHFFFAOYSA-N
Applicazioni
Note legali
Avvertenze
Danger
Indicazioni di pericolo
Consigli di prudenza
Classi di pericolo
Aquatic Chronic 3 - Flam. Liq. 2 - Muta. 2 - Org. Perox. E
Codice della classe di stoccaggio
5.2 - Organic peroxides and self-reacting hazardous materials
Classe di pericolosità dell'acqua (WGK)
WGK 1
Punto d’infiammabilità (°F)
42.8 °F - closed cup
Punto d’infiammabilità (°C)
6 °C - closed cup
Dispositivi di protezione individuale
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.