152765
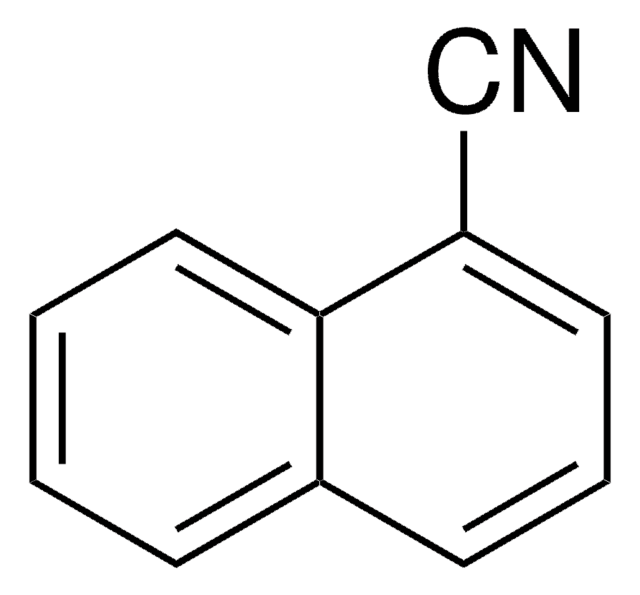
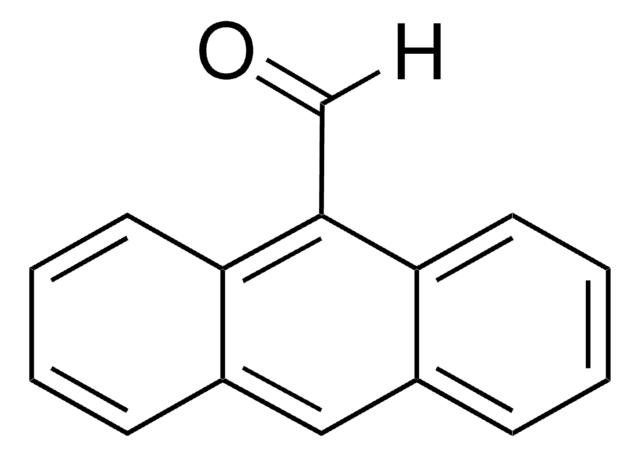
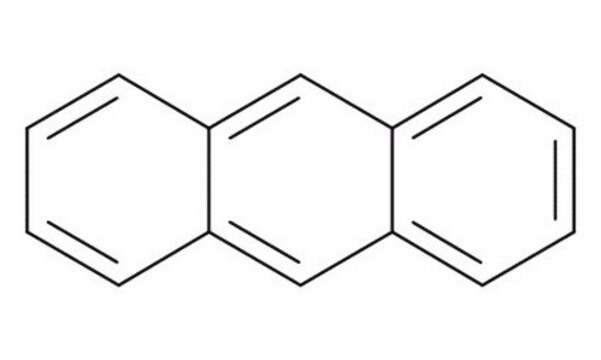
9-Anthracenecarbonitrile
97%
Sinonimo/i:
9-Cyanoanthracene
Autenticatiper visualizzare i prezzi riservati alla tua organizzazione & contrattuali
About This Item
Formula empirica (notazione di Hill):
C15H9N
Numero CAS:
Peso molecolare:
203.24
Numero CE:
Numero MDL:
Codice UNSPSC:
12352100
ID PubChem:
NACRES:
NA.22
Prodotti consigliati
Livello qualitativo
Saggio
97%
Stato
powder
Punto di fusione
173-177 °C (lit.)
Gruppo funzionale
nitrile
Stringa SMILE
N#Cc1c2ccccc2cc3ccccc13
InChI
1S/C15H9N/c16-10-15-13-7-3-1-5-11(13)9-12-6-2-4-8-14(12)15/h1-9H
KEQZHLAEKAVZLY-UHFFFAOYSA-N
Categorie correlate
Descrizione generale
The fluorescence excitation spectra of 9-anthracenecarbonitrile has been studied.
Applicazioni
9-Anthracenecarbonitrile was used to study the mechanism of charge separation within phenothiazine (PTZH) or phenoxazine (PXZH), and 9-cyanoanthracene(electron acceptor).
Avvertenze
Warning
Indicazioni di pericolo
Consigli di prudenza
Classi di pericolo
Acute Tox. 4 Dermal - Acute Tox. 4 Inhalation - Acute Tox. 4 Oral
Codice della classe di stoccaggio
11 - Combustible Solids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Dispositivi di protezione individuale
dust mask type N95 (US), Eyeshields, Gloves
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
Reika Kanya et al.
The Journal of chemical physics, 121(19), 9489-9497 (2004-11-13)
Fluorescence excitation spectra of the S(1)-S(0) origin band of 9-cyanoanthracene have been observed under a uniform electric field up to 200 kV/cm to explore pendular-state spectrum of an asymmetric-top molecule close to the strong field limit. The observed spectra exhibit
K Szarka et al.
Biochemistry, 40(49), 14806-14811 (2001-12-26)
It has been shown that one of the 12 serine residues within the 23 kDa segment of myosin subfragment 1 can be covalently modified with a fluorescent probe 9-anthroylnitrile (ANN) [Hiratsuka, T. (1989) J. Biol. Chem. 264 (30), 18188-18194]. To
N Shibata et al.
Journal of chromatography. B, Biomedical sciences and applications, 706(2), 191-199 (1998-04-29)
A new method for simultaneous determination of glucocorticoids (GCs) in plasma or urine by high-performance liquid chromatography (HPLC) with fluorimetric detection has been developed. Following extraction with ethyl acetate using a reversed-phase disposable cartridge, the six GCs [cortisol (F), cortisone
Franciszek K Główka et al.
Journal of chromatography. B, Analytical technologies in the biomedical and life sciences, 839(1-2), 54-61 (2006-03-23)
A new indirect RP-HPLC method was developed for determination of small, ng/ml, concentrations of triamcinolone (TMC) in human plasma, in presence of endogenous corticosteroids: cortisol (hydrocortisone, F), cortisone (E) and their metabolites, after administration of TMC in a free alcohol
O A Andreev et al.
Journal of muscle research and cell motility, 16(4), 353-367 (1995-08-01)
A serine residue located in the active site of myosin head (S1) was labelled by 9-anthroylnitrile, an amino group located in the central domain of S1 was labelled by 7-diethylamino-3-(4'-isothio-cyanato-phenyl)-4-methylcoumari n, a cysteine residue located near the C-terminus of S1
Global Trade Item Number
| SKU | GTIN |
|---|---|
| 152765-5G | 4061838740557 |
| 152765-25G |
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.